Apraglutide does not impact anti-tumor and immunosuppressive efficacy of conditioning chemotherapy in mice
Violetta Dimitriadou1, Mark Minden2.
1Translational Science, VectivBio, Basel, Switzerland; 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Background: Conditioning chemotherapy reduces tumor burden and provides immunoablation to prevent graft rejection with hematopoietic cell transplantation, but often induces mucosal barrier breakdown and mucositis. Apraglutide is a novel, long-acting synthetic glucagon-like peptide-2 (GLP-2) analog that protects the GI epithelium from chemotherapy-induced injury, improves survival, and allows better weight maintenance in mice undergoing chemotherapy. Preclinical studies aim to evaluate the impact of apraglutide on chemotherapy’s efficacy in reducing tumor load and inducing immunosuppression.
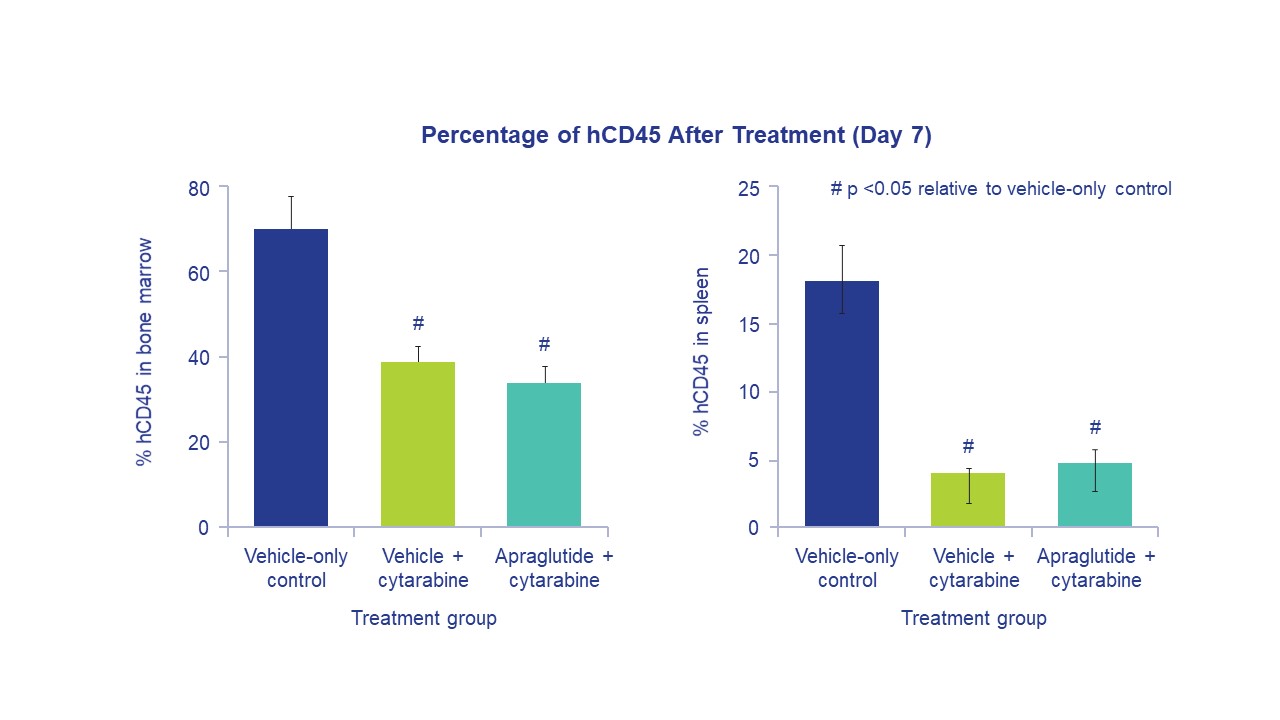
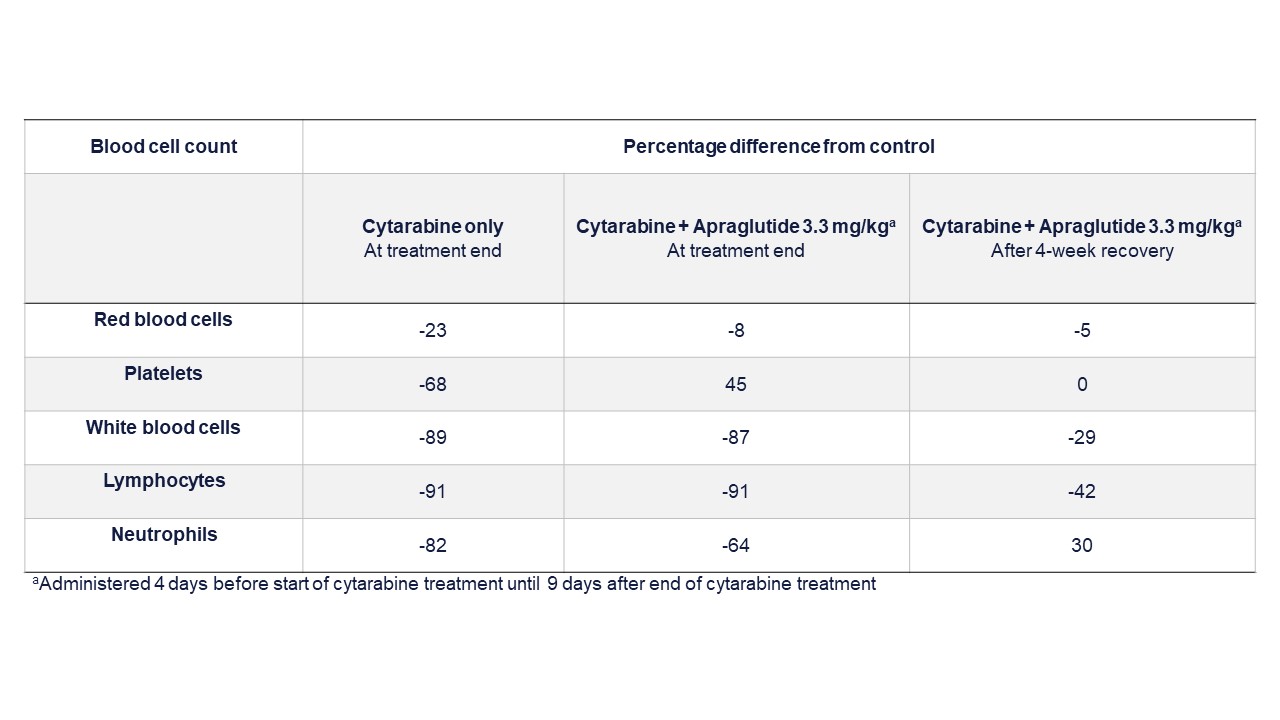
Methods: Study 1 assessed cytarabine’s antitumor effects in leukemic NOD/SCID mice. Apraglutide or vehicle was administered on Days -4 to 4. Cytarabine or vehicle was administered on Days 0-4. Bone marrow and spleen samples were collected on Day 7, and the percentage of hCD45+ cells was determined. Study 2 assessed the effect of apraglutide on cytarabine-induced immunosuppression and included three groups of Balb/c mice: (A) vehicle; (B) cytarabine on Days 5-9; (C) cytarabine on Days 5-9, concomitant apraglutide on Days 5-18. RBC, platelets, WBC, NEU, and LYMPH, were assessed. A cohort was allowed to survive for four weeks to evaluate the effect of apraglutide on immunosuppression recovery. Study 3 assessed the effect of apraglutide on melphalan-induced immunosuppression. Three groups of Balb/c mice were included: (A) vehicle; (B) melphalan on Day 9; (C) melphalan on Day 9, apraglutide pre-treatment on Days 1, 3, 5, 7 and continued as co-administration on Days 9, 11, and 13. WBC, NEU, and LYMPH were assessed.
Results: Study 1 showed that human leukemia cells reduction did not differ significantly between cytarabine-only and cytarabine + apraglutide and were significantly greater than in the vehicle-only group. The percentage of hCD45 in bone marrow after chemotherapy was 35.5±4 with cytarabine-only and 33.9±4.2 with cytarabine + apraglutide. A dramatic decrease in leukocytes at the end of the treatment period in Study 2 indicated that cytarabine-induced immunosuppression was not impaired by apraglutide co-administration (91% reduction in lymphocytes with both cytarabine + apraglutide and cytarabine-only). Apraglutide did not impact the recovery of hematological parameters four weeks after the end of treatment. Study 3 showed that melphalan elicited immunosuppression as evidenced by leukocyte decrease. Mice treated with melphalan, with or without apraglutide, had severe reductions in WBC and LYMPH vs. vehicle.
Conclusions: Pre- and concomitant apraglutide did not impair the efficacy of cytarabine in destroying human leukemia cells in vivo. Moreover, combination with apraglutide had no negative impact on cytarabine- or melphalan-induced immunosuppression. Apraglutide did not negatively impact the antitumor or immunosuppressive effects of cytarabine or melphalan.

right-click to download
