Apraglutide decreases severity of intestinal damage from acute gastrointestinal graft versus host disease (GI-GvHD) following allogeneic transplantation without impacting engraftment
Violetta Dimitriadou1, Geneviève Chabot-Roy2, Cindy Audiger2,3, Ianula Banu2, Jean-Sébastien Delisle2,4,5, Sylvie Lesage2,3.
1Translational Science, VectivBio, Basel, Switzerland; 2Centre de recherche de l’Hôpital, Maisonneuve-Rosemont Hospital, Montréal, QC, Canada; 3Département de microbiologie, infectiologie et immunologie, Université de Montréal, Montreal, QC, Canada; 4Département de médecine, Université de Montréal, Montreal, QC, Canada; 5Institut Universitaire en Hématologie Oncologie et Thérapie Cellulaire, Hôpital Maisonneuve-Rosemont - CIUSSS-EMTL, Montreal, QC, Canada
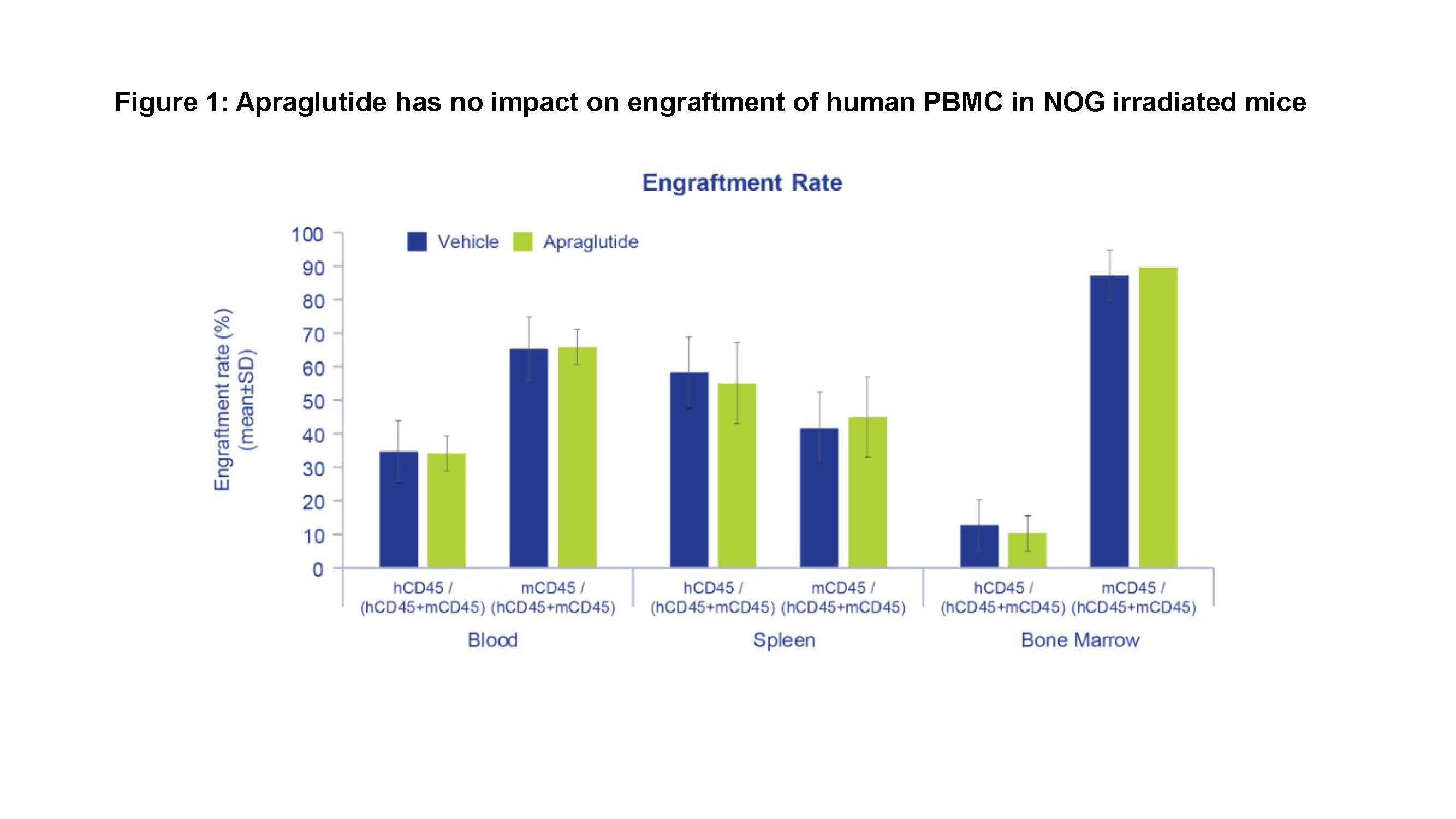
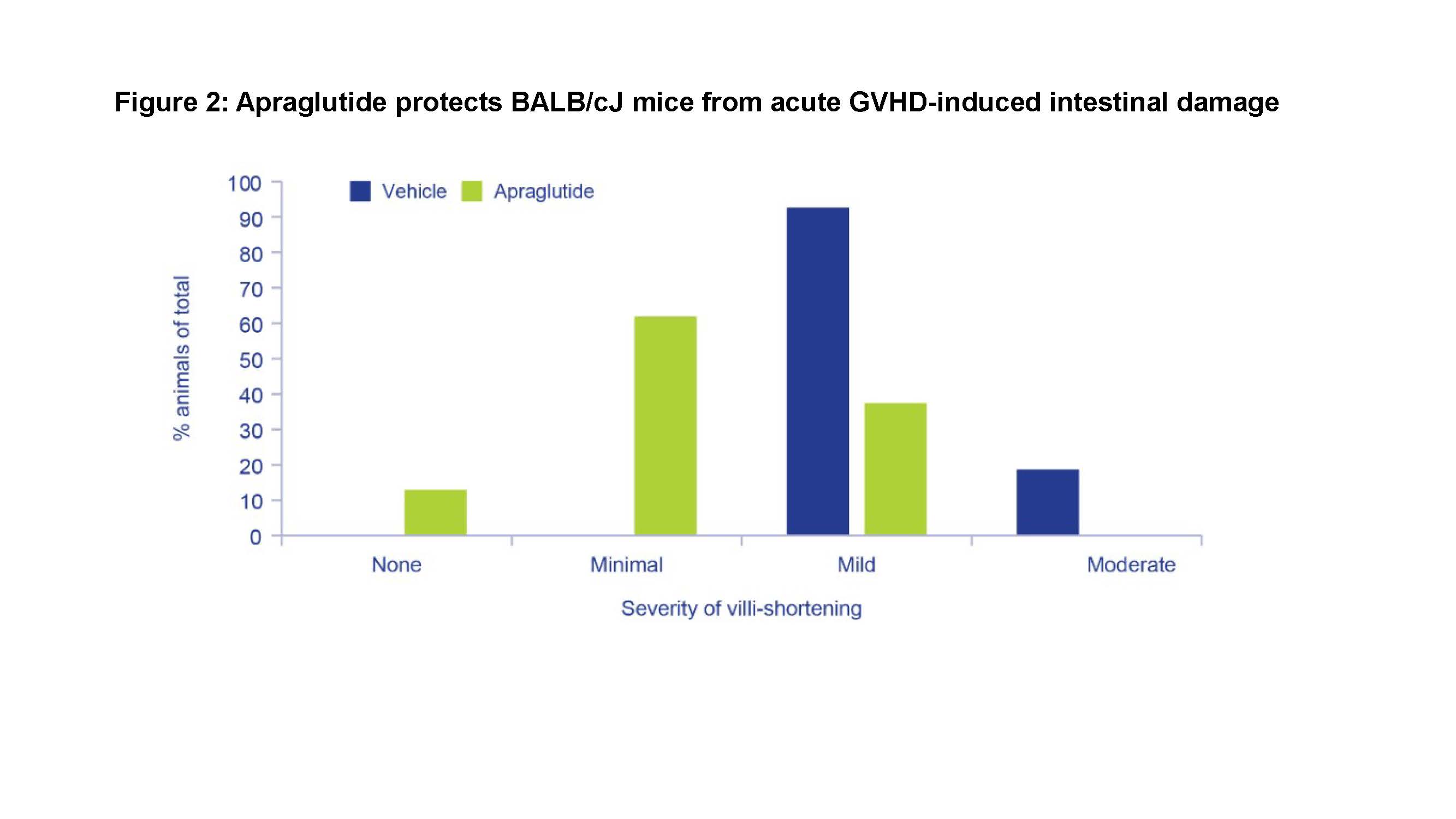
Background: The GI tract is a primary tissue system damaged by GvHD, leading to a compromised mucosal barrier, mucosal protein loss, and nutrient/fluid absorption failure. Glucagon-like peptide-2 (GLP-2) has demonstrated intestinotrophic effects, enhanced barrier function, and decreased intestinal permeability. Apraglutide, a novel, long-acting synthetic GLP-2 analog, represents a potential regenerative approach to GI GvHD prevention and treatment. Using two mice models of GvHD, we assessed the effects of apraglutide on engraftment and GI protection following irradiation and allogeneic transplantation.
Methods: In Study 1, total-body-irradiated (TBI) immunodeficient (NOG) mice (Day 0) were injected with human peripheral blood mononuclear cell (hPBMC; 3x107; Day 2) and treated with apraglutide 3.3 mg/kg or vehicle (Days -6 to 18). Engraftment rate was determined through CD45 expression (human vs. mouse) in blood, bone marrow, and spleen. In Study 2, TBI-induced intestinal damaged BALB/cJ mice received allogeneic transplantation from C57BL/6 strain and were treated with apraglutide (3.3 mg/kg) or vehicle (Days -9, -7, -5, -3 -1, +1, +3, +5, +7). Intestinal damage indicative of GvHD (histological changes, length, hemorrhage, inflammation), body weight, and survival were assessed.
Results: In study 1, hPBMC were successfully engrafted. The engraftment rate in blood, spleen, and bone marrow was not affected by apraglutide (range 22.2-47.6% at D20 in blood). hCD45+ cell infiltration was observed in the intestinal wall with no difference between apraglutide vs. vehicle. In study 2, lymphocyte engraftment was successfully achieved in both apraglutide- and vehicle-treated mice. Weight loss and median survival were similar in both groups, but apraglutide-treated mice had significantly higher overall survival vs. vehicle on Day +9 (40% vs. 0%, respectively; p=0.0134). Post-mortem histological examination revealed less mucosal degenerative/inflammatory changes (villous atrophy, mononuclear/neutrophilic cell infiltrate in the lamina propria/intra-cryptal epithelium, crypt necrosis) in apraglutide-treated mice vs. vehicle. Mean colon length in the apraglutide group (8.6±0.35 cm) was comparable to mice that did not undergo irradiation or transplantation (9.6±0.33 cm), whereas a significant reduction was apparent in the vehicle group (7.19±0.10 cm; p <0.05).
Conclusion: These results suggest that apraglutide treatment before allogeneic transplantation in immunodeficient mice does not affect engraftment rate. Furthermore, apraglutide showed a significant protective effect in TBI- and allogeneic-transplant-induced GvHD with reduced villi atrophy, less colon shortening, less severe intestinal damage, and showed a survival advantage. These findings support the beneficial role of apraglutide in reducing GI damage and limiting mortality from GvHD.

right-click to download
