Apraglutide treatment reduces chemotherapy-induced gastrointestinal (GI) damage in mice and preserves cellular integrity during chemotherapy
Violetta Dimitriadou1, Mark Minden2.
1Translational Science, VectivBio, Basel, Switzerland; 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Background: Chemotherapy-induced mucositis is a common condition caused by the breakdown of the mucosal barrier. Administration of exogenous glucagon-like peptide 2 (GLP-2) has been associated with reduced epithelial damage, decreased bacterial infection, and decreased mortality or gut injury in rodents with chemically induced enteritis. GLP-2 decreases chemotherapy-induced mucositis via inhibition of drug-induced apoptosis in the small and large bowel. Apraglutide is a novel, long-acting synthetic GLP-2 agonist that has been shown to promote intestinal growth and repair. Two preclinical studies aim to evaluate the efficacy of apraglutide (3.3 mg/kg) as pre-treatment or concomitant treatment in models of chemotherapy-induced intestinal damage with cytarabine or melphalan; both extensively used in hemato-oncology.
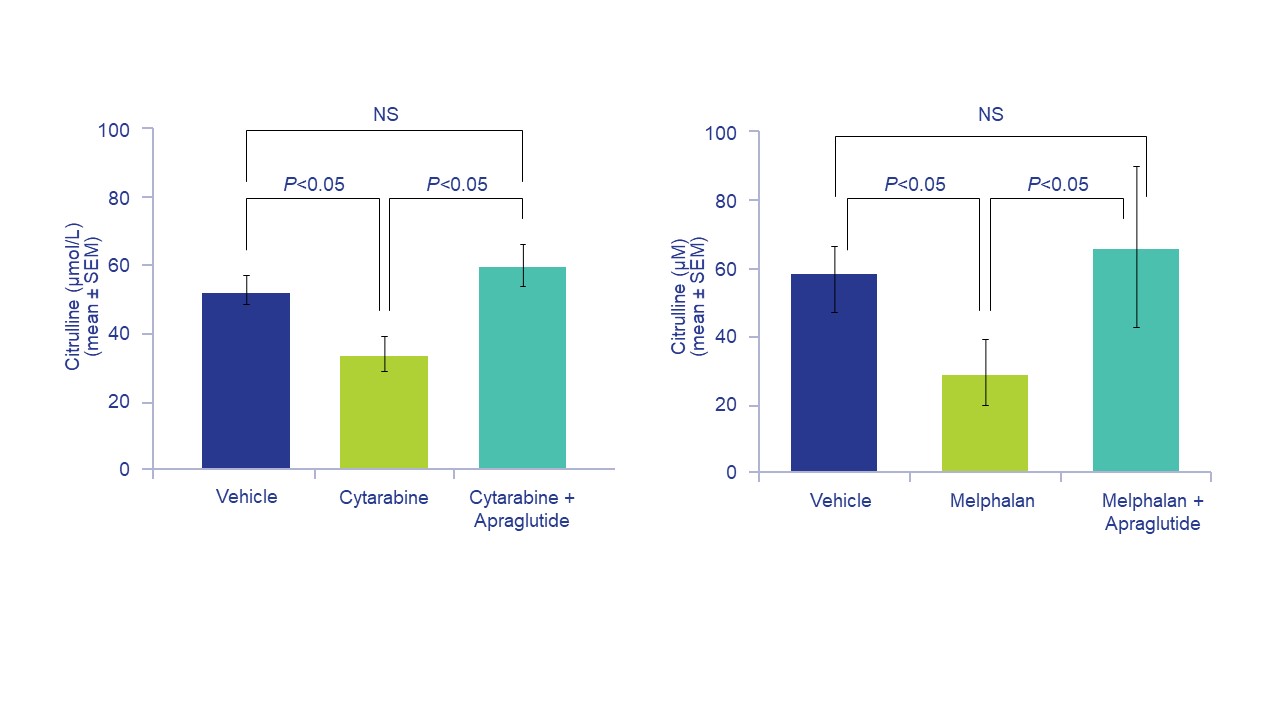
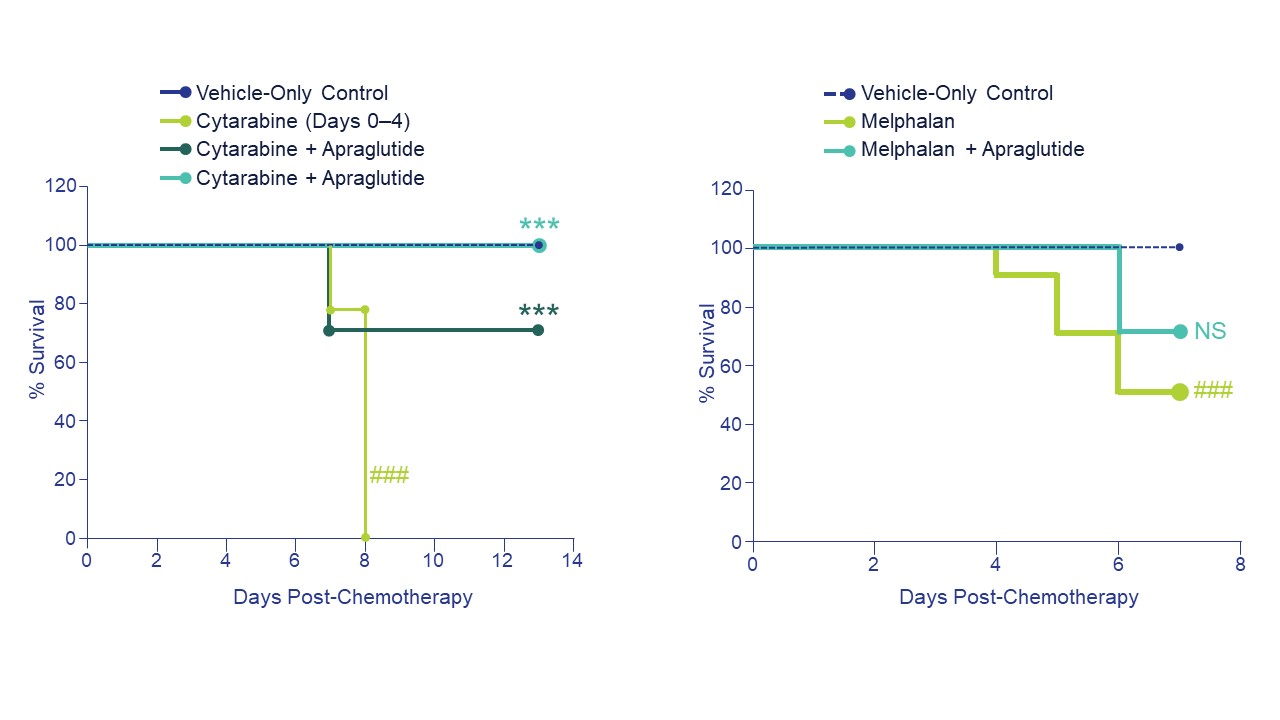
Methods: Study 1 included four groups of Balb/c mice: (A) vehicle only; (B) cytarabine on Days 5-9, no apraglutide given; (C) cytarabine on Days 5-9; concomitant apraglutide on Days 5-18; (D) cytarabine on Days 5-9; pre-treatment apraglutide on Days 1, 3, and continued as a concomitant treatment on Days 5, 8, 11, 14, and 17. Study 2 included three treatment groups of Balb/c mice: (A) vehicle only; (B) melphalan on Day 9, no apraglutide; (C) melphalan on Day 9; pre-treatment apraglutide on Days 1, 3, 5, 7 and continued as a concomitant treatment on Days 9, 11, and 13. In both models, mice that received the vehicle without any treatment served as controls. Intestinal tissue histology, body weight, survival, and plasma citrulline, a marker of total mucosal mass and intestinal growth, were assessed in both models.
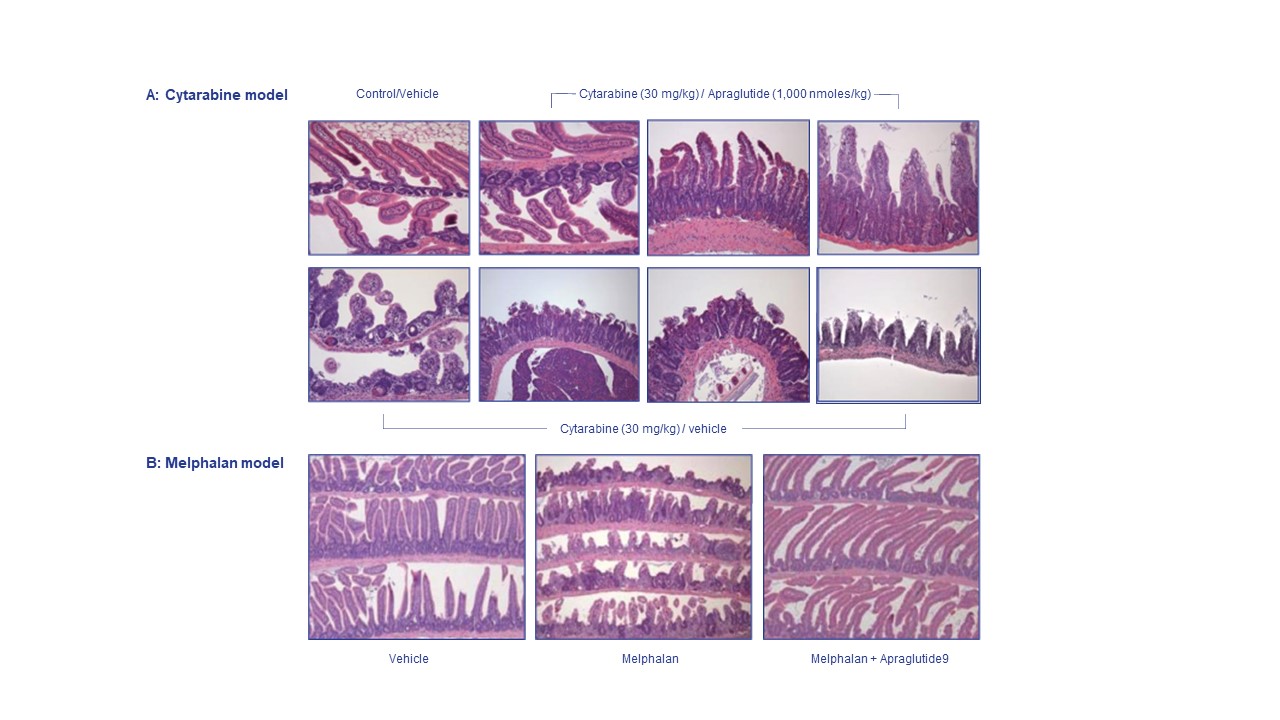
Results: Histological examination showed that the degenerative intestinal changes (villi and crypt atrophy) caused by cytarabine or melphalan were reduced by apraglutide co-administration, as demonstrated by similarities in tissue morphology between vehicle-treated and apraglutide-treated mice. In addition, the duodenum, ileum, and jejunum increased in weight with apraglutide. The intestinal protective effects of apraglutide were further supported by preserving plasma citrulline levels (a biomarker of intestinal mass): apraglutide-treated mice had similar levels to animals that did not receive chemotherapy. Apraglutide attenuated chemotherapy-induced weight loss and improved overall survival vs. vehicle-only or chemotherapy-only groups. The effects of apraglutide were optimal when it was administered as pre-treatment before chemotherapy.
Conclusion: Microscopic examination showed apraglutide protected GI epithelium structure from chemotherapy-induced injury, improved survival, and prevented severe body weight loss in mice undergoing chemotherapy. Apraglutide also maintained plasma citrulline levels, a marker of intestinal mass, comparable mice that did not undergo chemotherapy.

right-click to download
