Antibody response and molecular graft surveillance in kidney transplant recipients following SARS-CoV-2 vaccination
Nicole Ali1, Zoe Stewart1, Jake Miles2, Sapna Mehta1, Vasishta Tatapudi1, Bonnie Lonze1, Elaina Weldon1, Charles Dimaggio1, Jeanette Leonard1, Robert Montgomery1, Herati Ramin1.
1Transplant Institute, NYU Langone Health, New York, NY, United States; 2CareDx, CareDx, Brisbane, CA, United States
Introduction: Preliminary studies suggest that kidney transplant recipients (KTRs) show diminished humoral responses to SARS-CoV-2 vaccination. Although reports of allograft rejection around the time of SARS-CoV-2 vaccination have been rare, there is no recommended framework for monitoring for potential vaccine-related allograft injury. Here, we describe an approach for longitudinal assessment of immunogenicity and safety of SARS-COV-2 vaccination in KTRs.
Methods: KTRs eligible for SARS-CoV-2 vaccination were identified through medical records, beginning March 12, 2021. Baseline and weekly blood samples were collected for routine assessment, SARS-CoV-2 spike protein antibody titers, dd-cfDNA (AlloSure, CareDx) and gene expression profiling (GEP) (AlloMap, CareDx) for 12 weeks. HLA DSA testing was performed at baseline, 2 weeks after completion of vaccine doses and at week 12. Antibody response was defined as a 10-fold increase in total binding IgG titers.
Results: 49 KTRs were identified for analysis. Patient demographics are summarized in Table 1. 10 patients (20.4%) demonstrated a spike antibody response following completion of the vaccine series. Patients with a prior history of COVID-19 were more likely to mount a spike antibody response (n=8, 53.3%) compared to those with no reported history of COVID-19 (n=2, 5.8%). The odds ratio was 18.3 (95% CI 3.2, 105.0, p=0.0005). Median dd-cfDNA levels did not differ between pre- and post-vaccination (0.23% versus 0.21% respectively; Table 2). There was no significant difference between pre- and post-vaccination GEP scores (9.85 versus 10.4 respectively; Table 2). No patients developed clinically significant DSA, eGFR decline or allograft rejection following vaccination.
Conclusions: Quantitative antibody responses were strongly associated with a diagnosis of prior SARS-CoV-2 infection. Stability of eGFR, dd-cfDNA, GEP profiles and lack of allosensitization reinforce the safety profile of SARS-CoV-2 vaccination in KTRs. Further studies are needed to better understand immunogenicity in SARS-CoV-2 naïve individuals, including whether cellular responses are protective in the absence of humoral responses.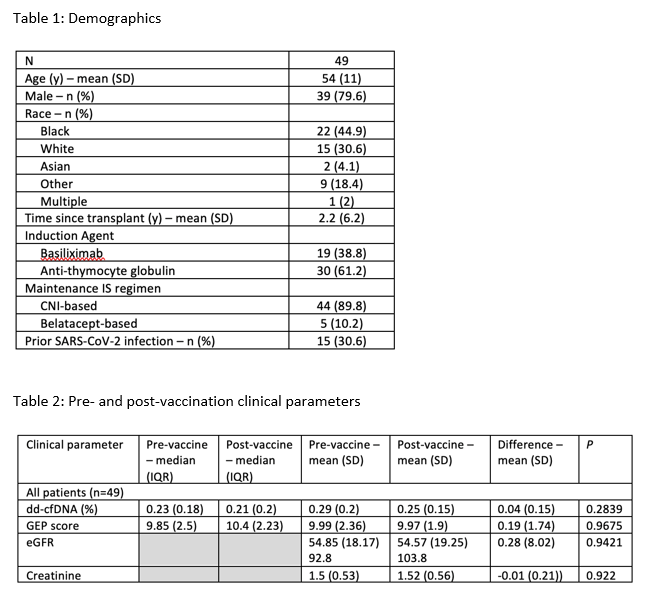

right-click to download
