Longitudinal surveillance of a kidney transplant recipient during pregnancy using quantification of fetal and donor-derived cell-free DNA
Jake Miles2, Vasishta Tatapudi1, Apra Mattoo1, Irfana Soomro1, Judith Benstein1, Nicole Ali1.
1Transplant Institute, NYU Langone Health, NY, NY, United States; 2CareDx, CareDx, Brisbane, CA, United States
Introduction: Following kidney transplantation, women of childbearing age often experience rapid restoration of fertility as the feedback mechanisms of the hypothalamic-pituitary-gonadal axis normalize. Although rates of rejection during pregnancy are comparable to the non-pregnant transplant population, subtle indications of allograft injury such as increases in serum creatinine or proteinuria can be masked by pregnancy related changes. Here, we describe a novel approach of allograft surveillance using quantification of fetal and donor-derived cell-free DNA (dd-cfDNA) throughout gestation and postpartum.
Methods: The patient was monitored longitudinally with dd-cfDNA (AlloSure, CareDx) pre-pregnancy, during gestation and postpartum. For this analysis, specific SNPs were selected that are heterozygous in kidney donor and homozygous (same allele) in recipient and fetal cells. An average minor allele frequency (MAF) was calculated from these selected SNPs. This average represents half of the expected donor cfDNA% due to Hardy-Weinberg principle based on Mendelian genetics. Hence, calculated average MAF was multiplied by factor of 2 to calculate the kidney donor percentage.
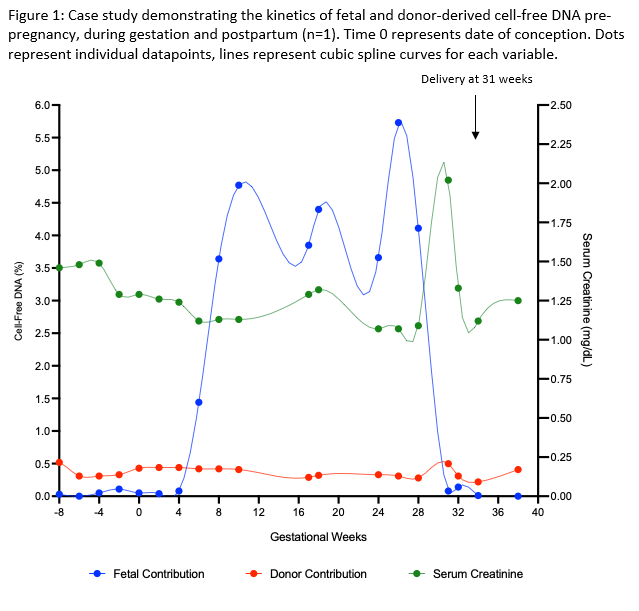
Results: A 39 year old female with a living related kidney transplant (transplanted September 2018) conceived in February 2021. The patient had a baseline creatinine of 1.4 mg/dl and median dd-cfDNA level of 0.44%. dd-cfDNA levels remained stable throughout pregnancy (median 0.42%, IQR 0.31-0.44%) and were consistent with pre-pregnancy baseline, in keeping with absence of allograft injury. The percentage of fetal cfDNA saw an exponential rise in the first trimester, plateaued in the second trimester and showed a rapid decay post-delivery. Allograft function remained stable throughout pregnancy until development of pre-eclampsia with an acute rise in serum creatine to 2.1 mg/dl, prompting delivery at 31 weeks (Figure 1).
Conclusions: The estimated percent of fetal fraction of cfDNA increased with gestational age and rapidly declined post-delivery. Throughout pregnancy, dd-cfDNA levels remained consistent with pre-pregnancy baseline in the absence of graft dysfunction, rejection, or clinical events. This case study suggests that longitudinal monitoring of fetal and dd-cfDNA contributions during pregnancy in kidney transplant recipients is a feasible, novel approach for allograft surveillance in this high-risk patient population.

right-click to download
