Kinetics of dd-cfDNA in kidney transplant recipients following SARS-CoV-2 vaccination booster administration
Nicole Ali1, Jake Miles2, Vasishta Tatapudi1, Apra Mattoo1, Irfana Soomro1, Judith Benstein1, Sapna Mehta1, Henry Neumann1, Fainareti Zervou1, Robert Montgomery1.
1Transplant Institute, NYU Langone Health, NY, NY, United States; 2CareDx, CareDx, Brisbane, CA, United States
Introduction: Evolving data suggests that booster vaccine doses enhance the immunogenicity of SARS-CoV-2 vaccination in solid organ transplant (SOT) recipients with higher IgG responses, neutralizing antibodies titers, and greater SARS-CoV-2–specific T-cell counts. However, antibody responses are attenuated by immunosuppression regimens and a significant proportion of patients continue to be at high risk for infection despite vaccination. Currently, there is no recommended framework for monitoring for potential vaccine-related immunological graft injury. Here, we describe kinetics of dd-cfDNA pre- and post-booster vaccination in kidney transplant recipients (KTRs), including dual organ recipients.
Methods: Electronic medical records were reviewed to identify KTRs that received a SARS-CoV-2 booster vaccine dose in 2021 and were monitored with dd-cfDNA (AlloSure, CareDx) pre- and post-vaccination. dd-cfDNA was collected as part of standard of care assessment. Pre-booster dd-cfDNA levels were defined as the most recent result prior to booster dose administration. Post-vaccination results were collected up to 30 days post-booster administration.
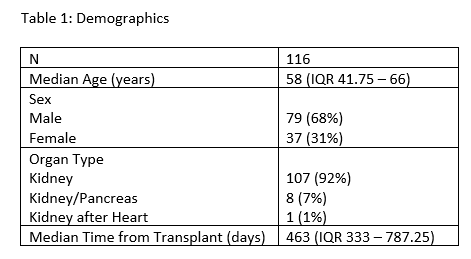
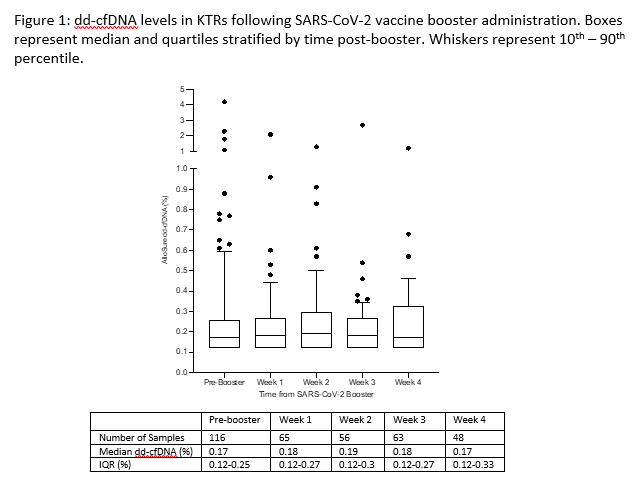
Results: 116 KTRs were identified for analysis. Patient demographics are summarized in Table 1. Median time from transplant to SARS-CoV-2 booster administration was 463 days (IQR 333 – 787.25 days, Table 1). Pre-booster dd-cfDNA levels were established a median of 9 days (IQR 2.25 – 16 days) prior to vaccination. The median level of dd-cfDNA pre-booster was 0.17% (IQR 0.12% – 0.25%). There was no significant difference in median levels of dd-cfDNA up to 30 days post-vaccine booster administration (Kruskal Wallis test with multiple comparisons, all p values >0.99, Figure 1). No adverse clinical events or acute rejection episodes were reported within 30 days of SARS-CoV-2 booster administration in this cohort.
Conclusions: Median dd-cfDNA levels were not impacted by SARS-CoV-2 vaccine booster administration, suggesting that patterns of subclinical injury that may potentiate inflammation, allosensitization or allograft rejection are unlikely in this setting. The stability of dd-cfDNA demonstrated here further reinforces the safety profile of SARS-CoV-2 vaccine booster administration in KTRs.

right-click to download
