
Escape from macrophage-mediated rejection by human surfactant protein (SP)-A
Chiyoshi Toyama1, Akira Maeda1, Shuhei Kogata1, Riho Yamamoto1, Takehisa Ueno1, Masafumi Kamiyama1, Yuko Tazuke1, Hiroshi Eguchi1, Hiroomi Okuyama1, Shuji Miyagawa1,2.
1Department of Pediatric Surgery, Osaka Univesity, Suita, Japan; 2 International Institute for Bio-Resource Research, Meiji University, Kawasaki, Japan
Introduction: Macrophage-mediated xenogeneic rejection is one of the important immunological obstructions that need to be overcome. Because macrophage is one of the main sources of proinflammatory cytokines and the fact that proinflammatory cytokines orchestrate a variety of inflammatory responses, the regulation of macrophages could result in solving the problem of xenogeneic rejection. We recently reported that membrane-type surfactant D (SP-D) on swine endothelial cells (SECs) suppresses macrophage-mediated rejection. Similar to SP-D, the carbohydrate recognition domain of surfactant protein-A (SP-A) induces inhibitory signals in effector cells. In this study, we examined the suppressive effect of SP-A on macrophage-mediated xenogeneic rejection.
Methods: Naïve SEC and SP-A-transfected SEC (SEC/SP-A) were co-cultured with THP-1 cells and cytotoxicity was evaluated. To investigate the effect on phagocytosis, human macrophages were co-cultured with SEC or SEC/SP-A, and the extent of phagocytosis and production of reactive oxygen species (ROS) were assessed by flow cytometry. The mRNA expression of inflammatory cytokines in macrophages was determined using RT-PCR. In addition, THP-1 cell with a luciferase reporter gene driven by an NF-kB response element (THP-1 Luc NF-kB) cells was used to verify their effects on the NF-κB transcription factors.
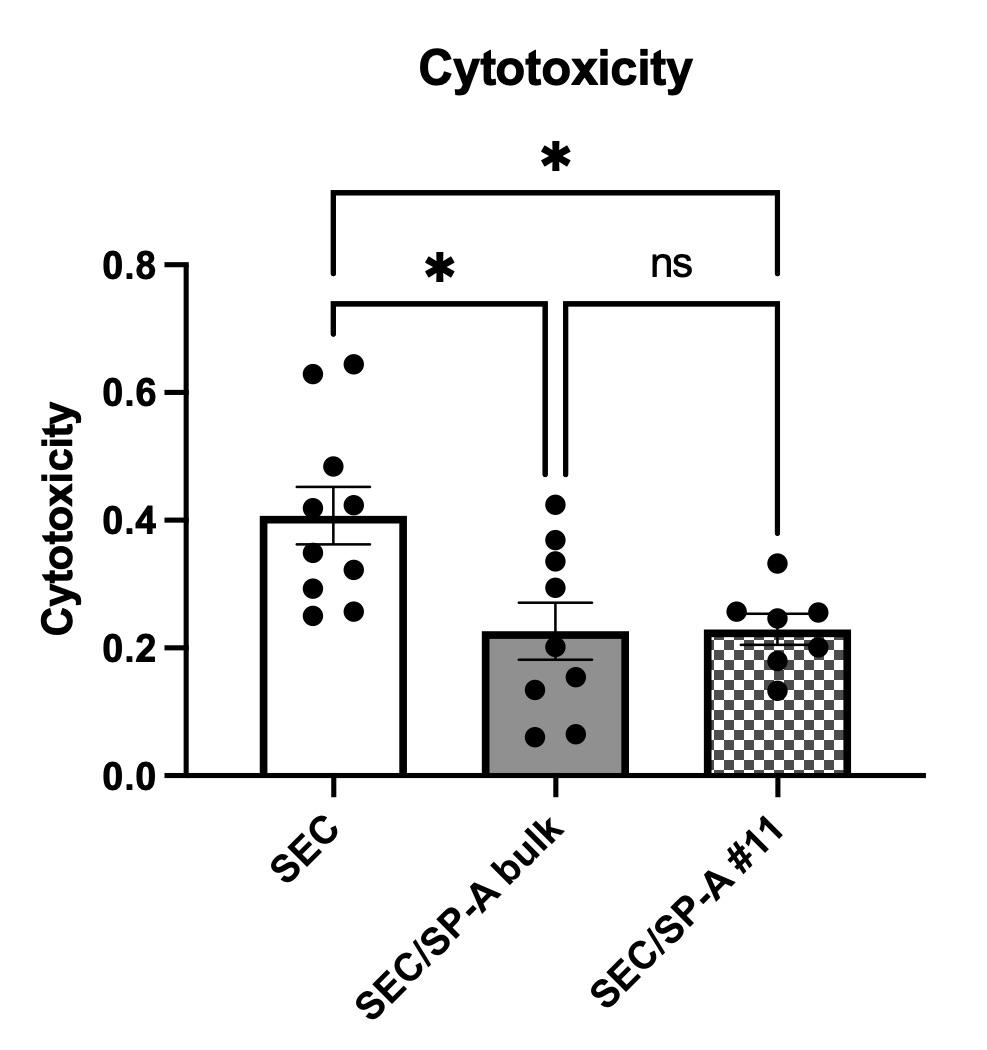
Results: The cytotoxicity of SEC/SP-A was significantly reduced compared to those of naïve SEC (40.7 vs 22.9%, p=0.025). The phagocytosis by macrophages against SEC was also significantly suppressed by SP-A on SEC (75.8 vs 33.0%, n=5, p=0.0010). {{AbstractFigure.2}}Co-culture of human macrophages with SEC/SP-A decreased ROS production (%MFI: 99.9 vs 79.5, p=0.013). The mRNA expression of TNFα was reduced in macrophages (1.160 vs 0.009, p=0.0022), whereas that of IL-10 was increased (1.357 vs 10.20, p=0.0007). The balance between iNOS and Arg-1 was significantly suppressed by CL-SPA (1.002 vs 0.4792, p = 0.0027), indicating that CL-SPA on porcine cells inhibits the differentiation of peripheral blood monocytes into inflammatory M1 macrophages. The NF-κB transcription was decreased in THP-1 Luc NF-kB which was co-cultured with SEC/SP-A compared to that in THP-1 Luc NF-kB with SEC (RLU: 1160 vs 983.8, p=0.0258).
Conclusion: The ectopic expression of human SP-A in porcine cells represents an attractive method for suppressing macrophage-mediated cytotoxicity. To further investigate the effects of SP-A on xenogeneic innate immune response in more detail, in vivo studies should be performed in the future.

right-click to download
