
Consequences of breakthrough COVID-19 infection in solid organ transplant recipients relative to non-immunosuppressed controls
Amanda Vinson1, Alfred J. Anzalone2, Jing Sun3, Ran Dai4, Gaurav Agarwal5, Stephen B. Lee6, Evan French7, Amy Olex7, Michael G. Ison8, Roslyn B. Mannon9.
1Medicine, Dalhousie University, Halifax, NS, Canada; 2Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, United States; 3Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States; 4Biostatistics, University of Nebraska Medical Center, Omaha, NE, United States; 5Medicine, University of Alabama at Birmingham, Montgomery, AL, United States; 6Medicine, University of Saskatchewan, Regina, SK, Canada; 7Virginia Commonwealth University, Richmond, VA, United States; 8Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, United States; 9Medicine, University of Nebraska Medical Center, Omaha, NE, United States
Introduction: The COVID-19 pandemic has had an immense impact on solid organ transplant (SOT) recipients. COVID-19 vaccination is remarkably effective in the general population; however SOT recipients have an impaired immune response and vaccine efficacy in this immunosuppressed population has been questioned. Most studies of vaccination in SOT recipients to date have primarily examined the immune response to vaccination, with less consideration of the clinical outcomes following breakthrough COVID infection.
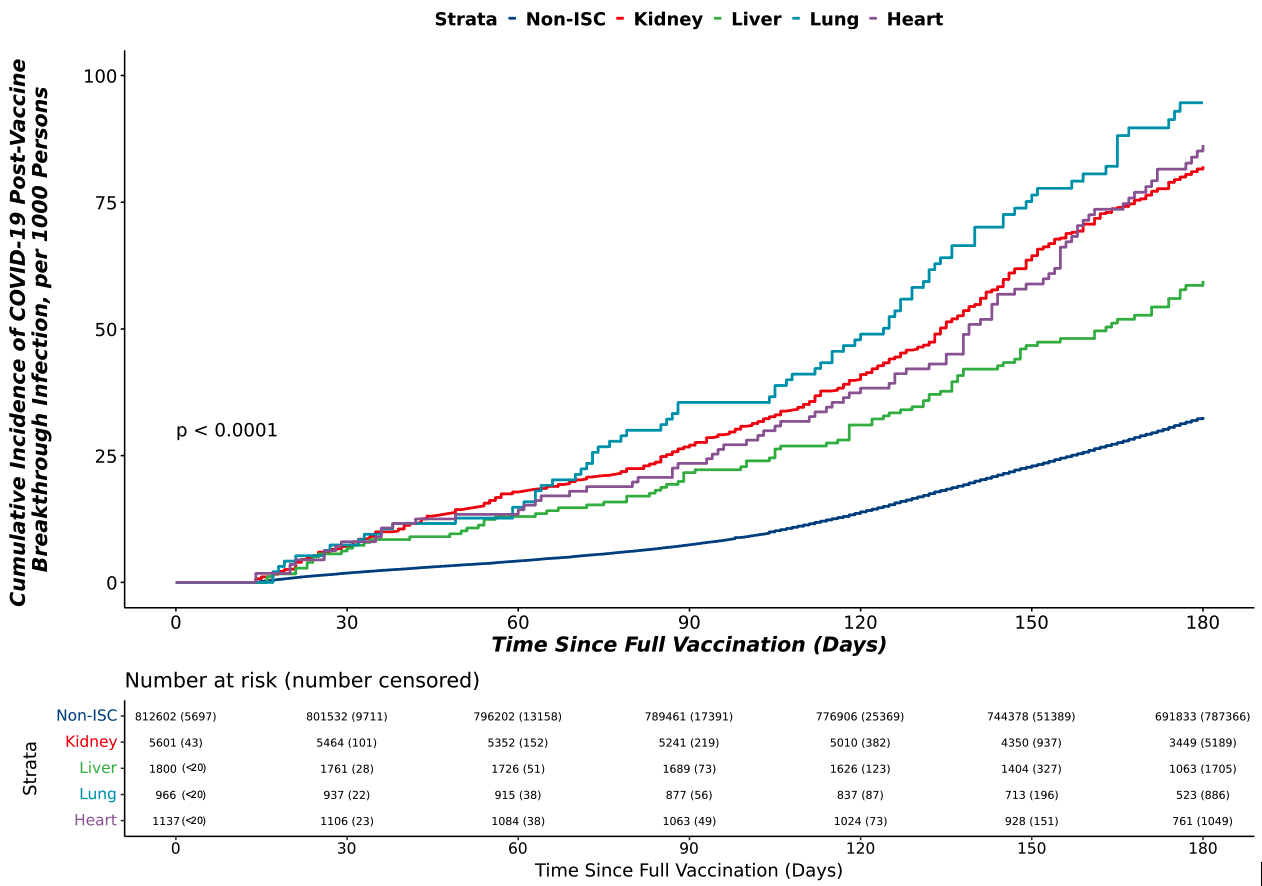
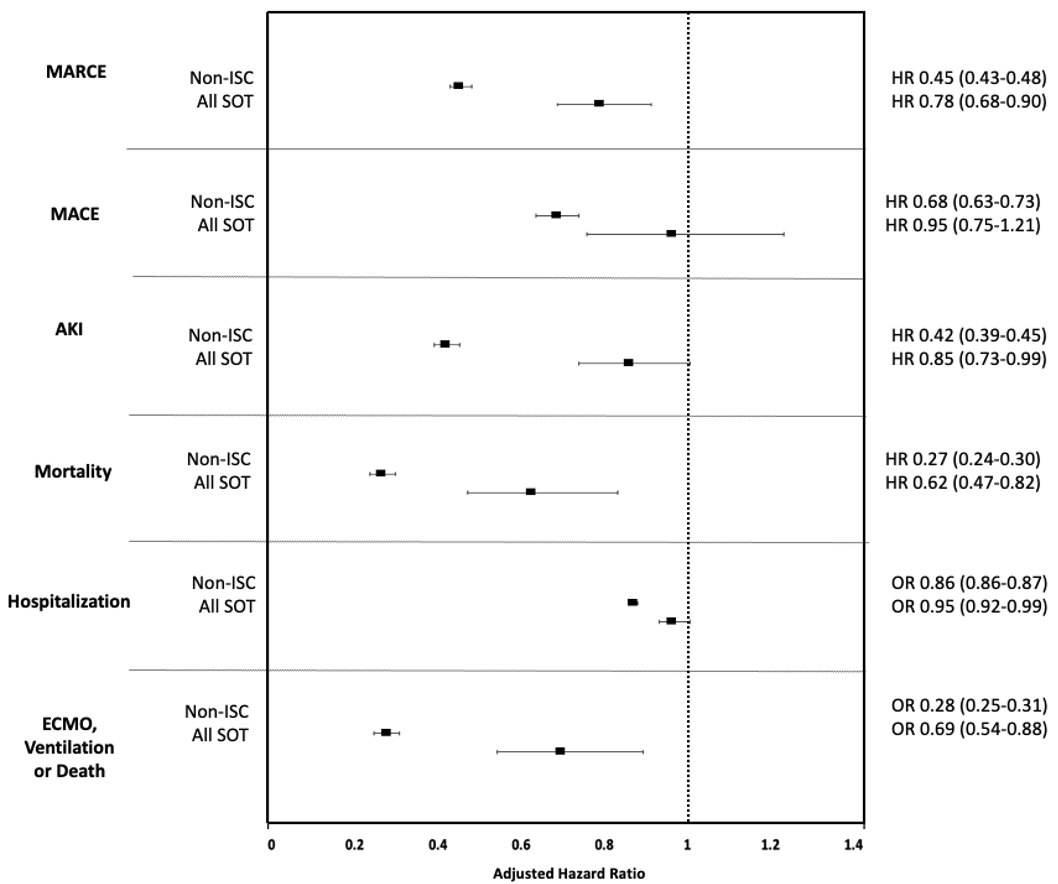
Methods: In a cohort of adult patients testing positive for COVID-19 prior to the Omicron wave (12/10/2020-12/18/2021), we used data from 37 sites across the United States using the National COVID Cohort Collaborative (N3C) to examine the efficacy of 2 doses of mRNA vaccination or 1 dose of Johnson & Johnson (VAX2) in a non-immunocompromised/immunosuppressed (ISC) population compared to SOT recipients. The cumulative incidence of breakthrough COVID-19 (BTCo) in the 6 months post VAX2 in the non-ISC population and in the SOT cohort by organ type (kidney, liver, lung, or heart) was demonstrated using cumulative incidence curves per 1000 persons. We assessed the risk of complications (major adverse renal or cardiac events (MARCE), major adverse cardiac events (MACE), acute kidney injury (AKI), mortality, hospitalization, or a composite of requiring ECMO, ventilation, or dying) in the 90-days post BTCo in VAX2 SOT recipients versus SOT with unconfirmed vaccination status (UVS) using multivariable Cox proportional hazards and logistic regression as required. This analysis was repeated in the non-ISC population for comparison.
Results: Over the study period, following VAX2, BTCo occurred in 818 (11.5%) SOT recipients and 38,401 (5.7%) non-ISC patients. Median time from vaccination to BTCo was 117 days (IQR 68-145) in the SOT cohort and 127 days (IQR 93-154) in the non-ISC cohort. Lung transplant recipients had the highest cumulative incidence of BTCo, and after non-ISC, and liver transplant recipients had the lowest (Figure 1). The greatest relative benefit with vaccination for both non-ISC and SOT cohorts was in BTCo mortality (HR 0.27, 95% CI 0.24-0.30 for non-ISC and HR 0.62, 95% CI 0.47-0.82 for SOT relative to UVS), and in the composite of needing ECMO, ventilation, or dying (OR 0.28, 95% CI 0.25-0.31 for non-ISC and HR 0.69, 95% CI 0.54-0.88 for SOT relative to UVS), Figure 2.
Conclusion: While the relative benefit of vaccine was less in SOT than non-ISC, SOT patients still exhibited significant benefit with vaccination. Although this study demonstrates moderate benefit in reducing major complications after BTCo in SOT recipients, immunosuppressed patients must remain vigilant of their risk and continue to minimize exposure.
