
Incidence and severity of COVID-19 infections among triple-vaccinated solid organ transplant recipients during the Omicron variant surge in the United States (12/25/2021-3/1/2022)
Jennifer Alejo1, Teresa PY Chiang1, Laura Bowles Zeiser1, Jake D Kim1, Jonathan Mitchell1, Robin K Avery1, Aaron AR Tobian3, Allan B Massie4, William A Werbel2, Dorry L Segev4.
1Surgery, Johns Hopkins, Baltimore, MD, United States; 2Medicine, Johns Hopkins, Baltimore, MD, United States; 3Pathology, Johns Hopkins, Baltimore, MD, United States; 4Surgery, NYU Langone Health, New York, NY, United States
Johns Hopkins COVID-19 Vaccine Study Team.
Introduction: Incidence and severity of SARS-CoV-2 Omicron variant infections among vaccinated solid organ transplant recipients(SOTRs) is unknown. We surveyed SOTRs from our national observational cohort (United States) during the Omicron wave(12/25/2021-3/1/2022).
Methods: 1467 triple-vaccinated SOTRs who reported no prior positive COVID-19 test were surveyed regarding new COVID-19 infections after 12/25/2021. A subgroup underwent serologic testing for anti-spike antibodies between 11/26/2021 and 3/7/2022 on two clinical assays (Roche Elecsys anti-receptor binding domain [RBD] assay or Euroimmun anti-S1 assay). If SOTRs reported a new COVID-19 infection, the most recent pre-infection antibody result was used. Otherwise, the preceding antibody level closest to 12/25/2021 was used.
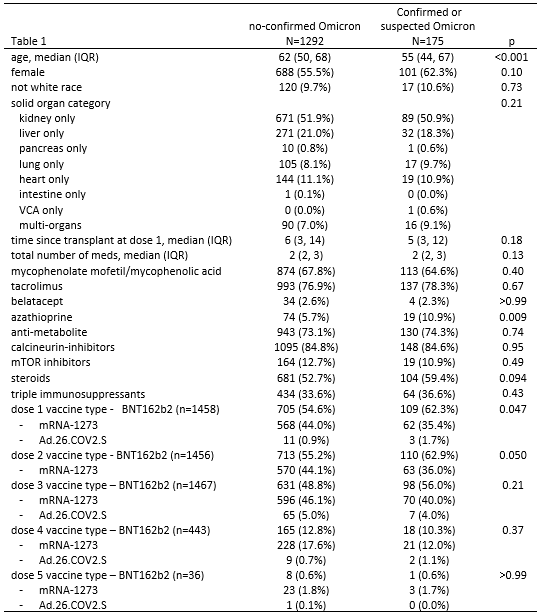
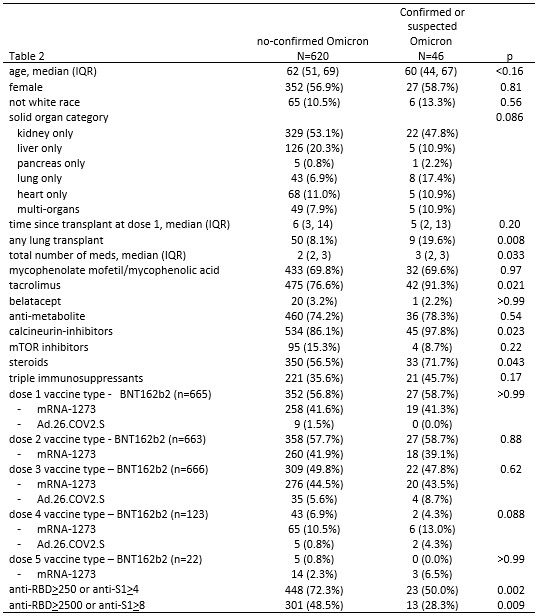
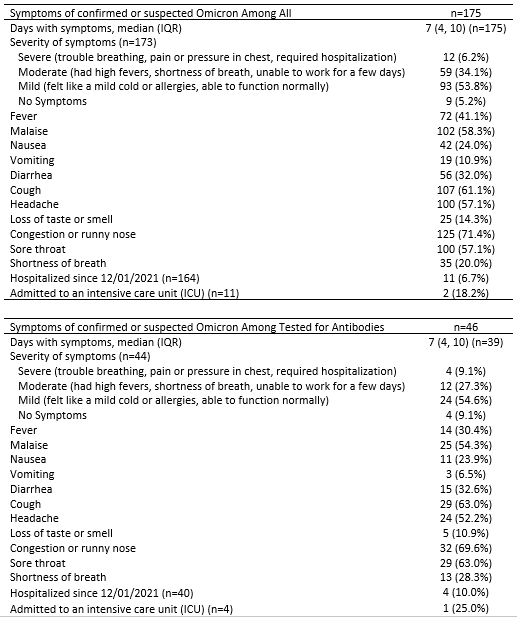
Results: 1467/1801 eligible SOTRs responded to the survey (81.6%) and 666 underwent serologic testing. 175/1467(12%) reported suspected or test-confirmed COVID-19 infection during the Omicron wave; 150/175(86%) reported having a positive PCR or home COVID-19 test (Omicron-confirmed). SOTRs who were Omicron-confirmed were younger (median [IQR] 55 [44-67] vs. 62 [50-68], p<0.001) and more likely to be using azathioprine (11% vs. 6%, p=0.009) (Table 1). Regarding illness severity, 11/164(7%) reported hospital admission for COVID-19, 59/173(34%) reported high-fevers or dyspnea, 93/173(54%) reported mild cold-like symptoms, and 9(5%) were asymptomatic. Symptoms included nasal congestion(71%), cough(61%), malaise(58%), headache(57%) and sore throat(57%)(Table 3A). Two SOTRs died during the study period; family remembers reported that these deaths were secondary to COVID-19 infection. Among the 666 SOTRs who underwent serologic testing, 46/666(7%) were Omicron-suspected or confirmed; 37/46(80%)tested positive via PCR or home test. The 46 Omicron-suspected or confirmed were more likely to be lung transplant recipients(20% vs. 8%, p=0.008), with greater number of immunosuppression medications(median, [IQR] 3 [2-3] vs. 2 [2-3], p=0.03), including tacrolimus(91% vs. 77%, p=0.02), steroids(72% vs.57%, 0.04), and calcineurin inhibitors(98% vs. 86%, p=0.02). 39/46 reported symptom duration of median (IQR) 7(4-10) days, 4/40 reported hospital admission, and 1/4 to an intensive care unit (Table 3B). 37/46(80%) of Omicron-suspected or confirmed and 534/620 (86%) of no-Omicron (p=0.3) were positive for anti-spike antibodies before the Omicron wave. 50% of Omicron-suspected or confirmed and 72% of no-Omicron SOTRs had anti-RBD anti-RBD>250 U/mL or anti-S1>4 AU (p=0.002)(Table 2).
Conclusion: Test-confirmed COVID-19 infections were reported in 5.7% of vaccinated SOTRs during the Omicron wave. COVID-19 infection appeared correlated with lower pre-Omicron wave antibody levels. These findings highlight the importance of layered prevention strategies for the immunocompromised including social distancing, masking, vaccination and passive immunity strategies.
This work was supported by the Ben-Dov family, the Trokhan Patterson family, grants T32DK007713 (Dr. Alejo), K01DK101677 (Dr. Massie), and K23DK115908 (Dr. Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases; grant K24AI144954 (Dr. Segev) from the National Institute of Allergy and Infectious Diseases; and grants U01AI138897 and K23AI157893 (Dr. Werbel).
