Keith S Hansen, United States has been granted the Young Investigator Congress Scientific Award

Eliminating second warm ischemia in order to address the organ shortage, increase transplant longevity, and enable minimally invasive kidney transplantation: ex-vivo validation of a kidney anastomosis facilitation and cooling device developed via the biodesign process
Keith Hansen1, James Gardner2.
1Department of Surgery, UCSF, San Francisco, CA, United States; 2Division of Transplantation, UCSF, San Francisco, CA, United States
Introduction: Kidneys are particularly susceptible to anoxic damage and ischemia due to their aerobic metabolism. Hypothermia protects against anoxia by reducing the energy dependent metabolic activities and is optimally achieved at 1-2oC for cold storage and 4-8oC for machine perfusion during transportation. There are no effective methods to prevent warming during implantation. The warming of a donor kidney during the vascular anastomosis of a transplant i.e., second warm-ischemia time (SWIT), is independently associated with higher rates of delayed graft function, premature graft failure, and the discard of high-risk kidneys. SWIT is protracted in patients with complex anatomy, obesity, and in minimally invasive transplantation. Elimination of SWIT via intra-operative thermal regulation has the potential to increase the donor pool and proffer significant cost-savings.
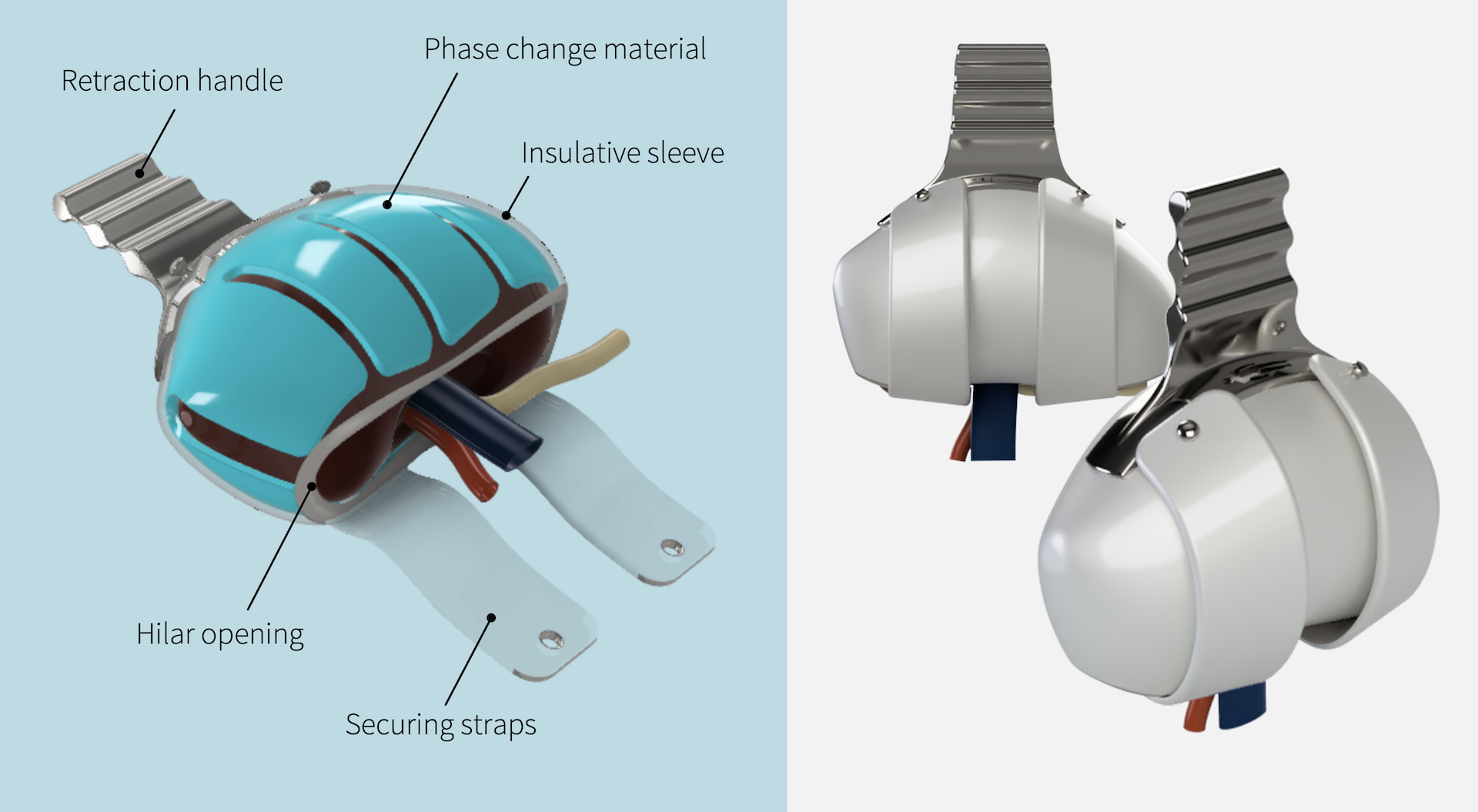
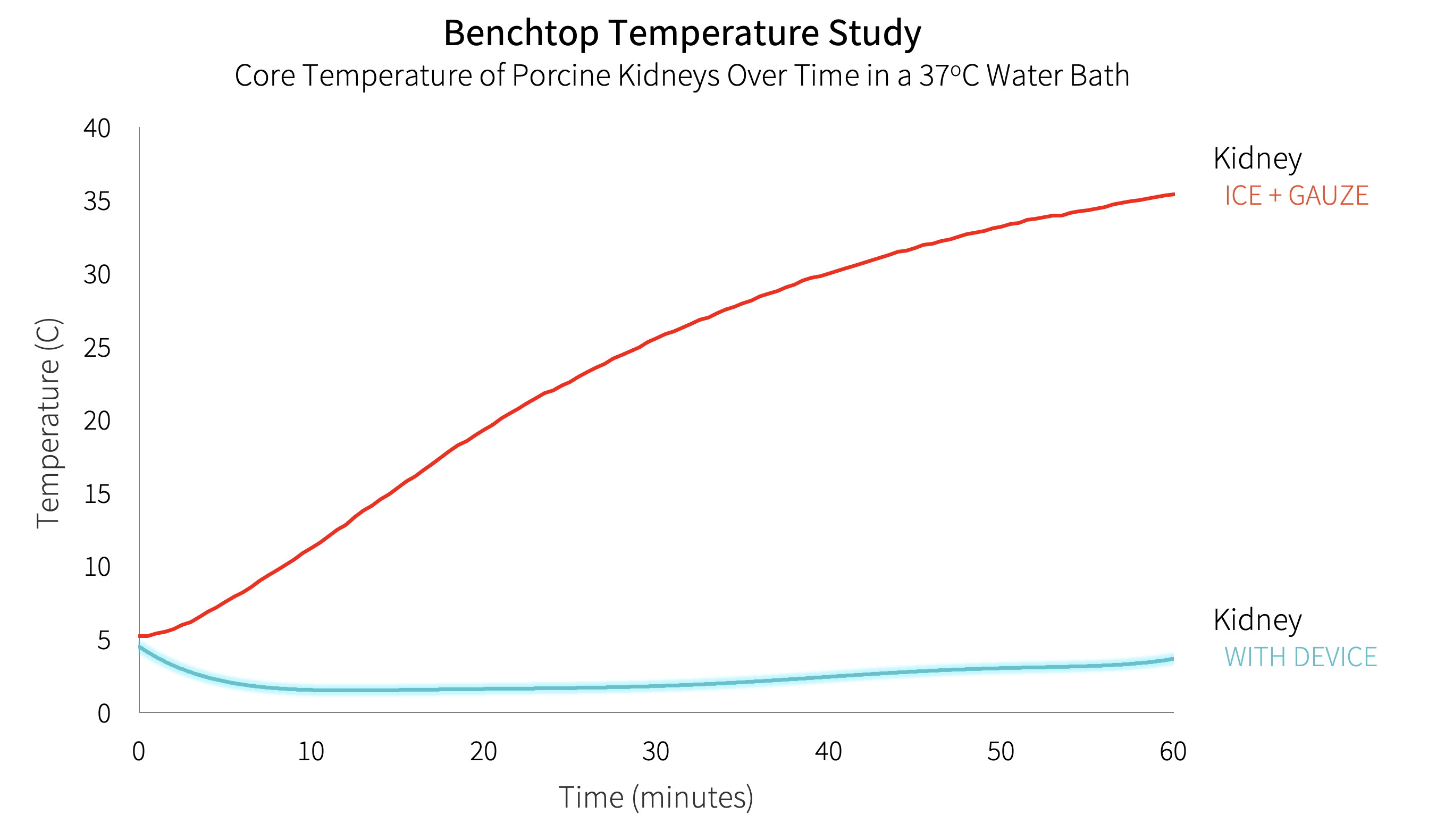
Method: The objective biodesign process was used to identify this clinical need through observation, develop a needs statement, and filter the need against others. A needs-specification document was developed outlining stakeholder value propositions, health-economic value, and existing intellectual property. ASTS surgeons (n=185) and organ-preservation specialists were surveyed to develop the needs-criteria for a device. The invention step resulted in several embodiments that addressed the clinical need, population, and outcome outlined in our needs statement. Following objective concept selection, a prototype device applied to the kidney immediately prior to anastomosis was built using stretchable hydrogel and phase-change gel. Adult porcine kidneys were used to test the device in a validated retroperitoneal-model placed within a water bath at 37oC (98.6oF). Core temperatures were monitored using implanted probes at 30 second intervals. Time to reach the maximum ischemic threshold of necrosis (15oC, 59oF) was compared to an ice + gauze control.
Results: A single-use device to facilitate the vascular anastomosis and eliminate SWIT was developed. The needs-criteria were addressed with a retraction handle, no tubing, a low profile, and a flexible material to accommodate variable kidney sizes. The device-covered kidneys (n=3) did not reach the ischemic threshold at the 60-minute cutoff and remained below 6oC compared with the ice + gauze covered control kidneys (17±1.8 minutes, n=3, p <0.001).
Conclusion: A breakthrough designated medical device to facilitate the vascular anastomosis and effectively eliminate second-warm-ischemia time was successfully developed. Device-covered kidneys remained well below the ischemic threshold of necrosis for a duration exceeding 95% of vascular anastomoses. Use of this device will enhance the surgical workflow and enable minimally-invasive transplantation and has the potential to significantly impact rates of delayed graft function, organ longevity, and organ acceptance practices.
Byers Center for Biodesign, Stanford University. UCSF Catalyst Program.
