
Cost-effectiveness of using a kidney anastomosis facilitation and cooling device to eliminate second warm ischemia time
Keith S Hansen1, Cynthia Yock2, Jan Pietzsch2.
1Department of Surgery, UCSF, San Francisco, CA, United States; 2Biodesign, Stanford University, Palo Alto, CA, United States
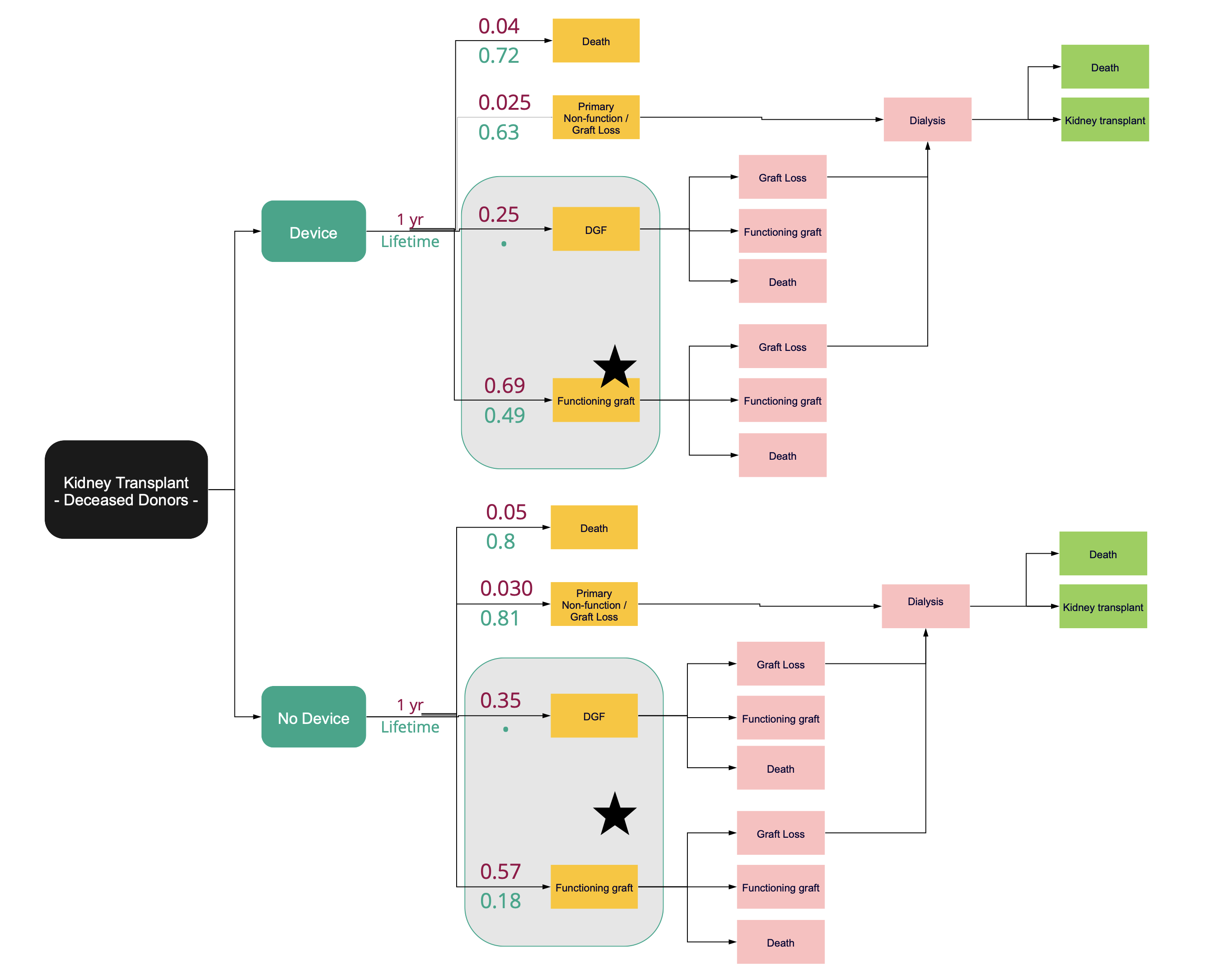
Introduction: The warming of a donor kidney during the vascular anastomosis of a transplant i.e., second warm-ischemia time (SWIT), is independently associated with higher rates of delayed graft function, premature graft failure, and the discard of high-risk kidneys. Existing literature shows that shortened SWIT and intra-operative cooling of a renal allograft during SWIT cumulatively reduces the incidence of delayed graft function in deceased donor kidney transplant recipients from a baseline rate of 35% to 25%, 5-year graft loss from 23% to 16%, and increases the number of transplantable kidneys by reducing the cumulative (first plus second) warm ishcemia time in deceased after circulatory death (DCD) donor kidneys. Cost effectiveness of a breakthrough anastomosis facilitation and SWIT eliminating cooling device was evaluated with a Markov Model.
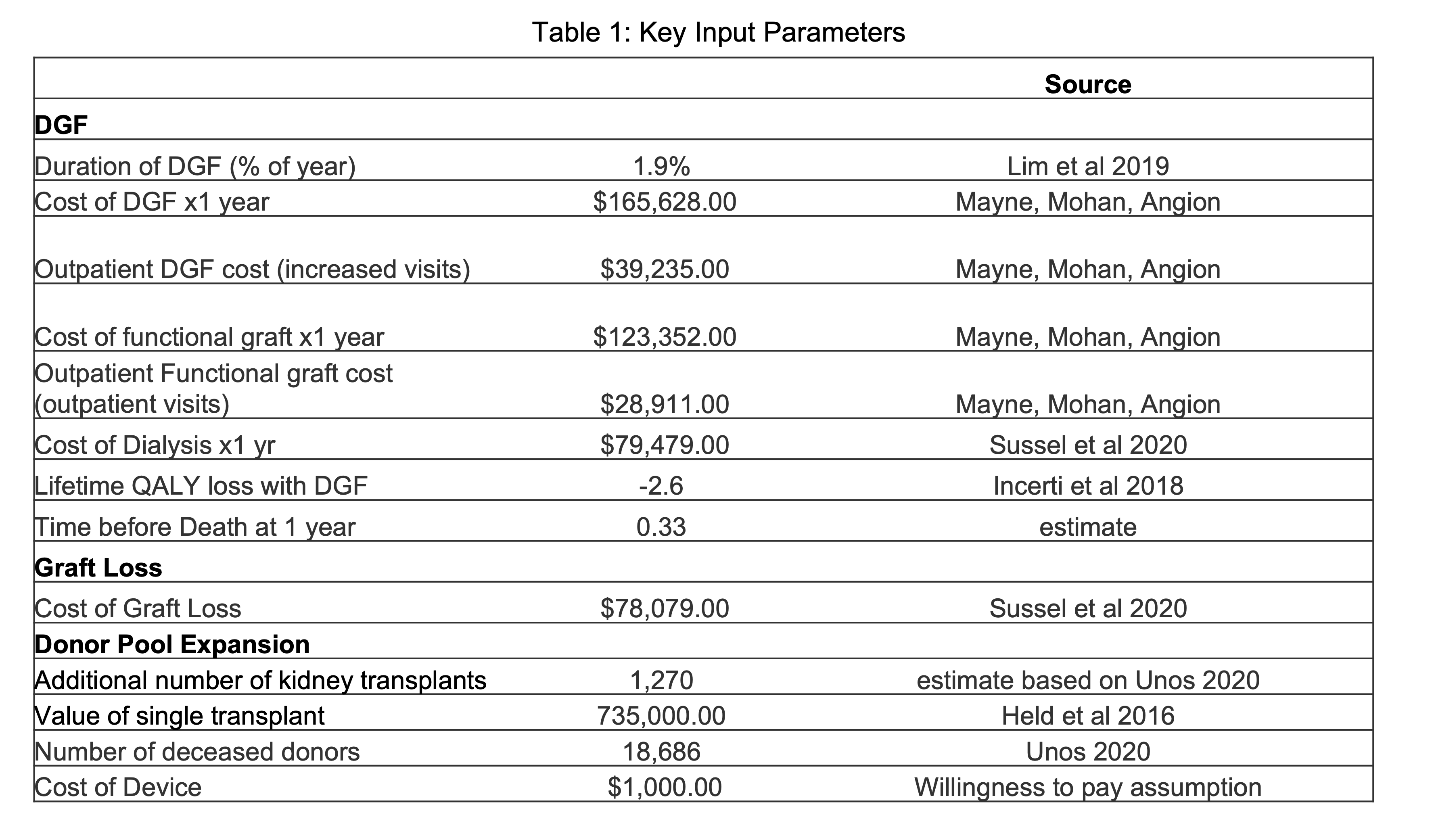
Methods: A state-transition model was used to predict the ff!ect of intra-operative cooling and standard of care on 1-year and lifetime probabilities of having a functioning graft, delayed graft function, graft loss with return to dialysis, and death. We adopted a societal perspective and estimated an incremental-cost-effectiveness ratio in U.S. dollars per quality adjusted life year (QALY).
Results: Intra-operative allograft cooling of deceased donor kidneys provides a 0.01 QALY improvement and $4,800 in savings per patient at 1-year, and over a lifetime, provides 0.26 QALYs and $12,200 in savings per patient. In both the 1-year and lifetime estimates, the ICER is dominant with the cooling device. The total cost savings, not including QALY gains to the system in this group, is $228 million. Including cost savings of the projected increase in organ utilization validated via a surgeon survey (n=175) resulted in $1.18 billion in savings over the lifetime of an annual cohort of transplant recipients. The 95% credible interval for ICER was cost-saving to $39,000 per quality adjusted life year.
Conclusion: The model suggests that intra-operative cooling of a deceased donor renal allograft, over a wide range of assumptions, is a cost effective strategy for transplantation that would result in significant cost savings and lower morbidity and mortality. Use of a breakthrough designated device to eliminate intra-operative warming over a 60 minute period and thereby reduce rates of delayed graft function, increase organ longevity, and change organ acceptance practices would lead to significant cost savings.
Byers Center for Biodesign, Stanford University.
