Long-term outcome and complications in pediatric ABO-incompatible living donor kidney transplantation with rituximab induction: a multi-centre investigation from the nordic paediatric renal transplantation study group
Felicia Kjaernet1,2, Anna Bjerre3,4, Zivile Bekassy5, Amir Sedigh9, Helle Thiesson6, Hjördis Thorsteinsdottir3, Anna Varberg Reisaeter8, Susanne Westphal7, Anders Åsberg8, Helena Genberg1,2.
1Dept of Transplantation Surgery, Karolinska University Hospital, Stockholm, Sweden; 2Div of Transplant Surgery, CLINTEC, Karolinska Institutet, Stockholm, Sweden; 3Division of Paediatric and Adolescent Medicine, Olso University Hospital, Oslo, Norway; 4Institute of Clinical Medicine, University of Oslo, Oslo, Norway; 5Dept of Pediatric Nephrology, Lund University, Lund, Sweden; 6Dept of Nephrology, University of Southern Denmark, Odense, Denmark; 7Dept of Pediatrics, University of Gothenburg, Gothenburg, Sweden; 8Dept of Transplantation Medicine, Olso University Hospital, Oslo, Norway; 9Dept of Surgical Sciences, Transplantation Surgery, Uppsala University, Uppsala, Sweden
Nordic Paediatric Renal Transplantation Study Group (NPRTSG).
Introduction: ABO-incompatible (ABOi) living donor kidney transplantation (LDKT) in adults has become routine practice at many centers worldwide. Pediatric ABOi LDKT has nonetheless remained a relatively rare event and reports on outcome following pediatric ABOi LDKT using rituximab induction are few. With the aim of evaluating long-term results and complications of pediatric ABOi LDKT, using a protocol based on antigen-specific immunoadsorption and rituximab, we undertook a retrospective multi-center study within the Nordic Paediatric Renal Transplantation Study Group (NPRTSG), comparing pediatric ABOi LDKT with ABO-compatible (ABOc) pediatric kidney transplantations with both living and deceased donors.
Material & Methods: Data was retrieved from the Scandiatransplant registry, the NPRTSG registry and medical records. In total, 6 out of 11 NPRTSG centers performed pediatric ABOi kidney transplantation (recipient age <16 years) during the study period (2003-2018). Data is currently available for 337 patients: 32(9,5%) ABOi LDKT, 73 (21,7%) ABO-compatible (ABOc) deceased donor kidney transplantations (DDKT) and 232 (68,8%) ABOc LDKT. A case-cohort analysis of graft survival, patient survival, complications and measured GFR was undertaken.
Results: Mean follow-up was 9.2 years (±4.6 years). Patient survival at 10 years was 100% in the ABOi group, 97.2% in ABOc LDKT group and 93.5% in the DD group (p=ns). Graft survival was higher in the ABOi group compared with the other two groups (Figure 1.). The length of first hospitalization did not differ (mean 19.6 ± 12.9 days), neither did the incidence of any medical, surgical, or infectious complication during first stay (52.1% overall) (p=0.68). More episodes of acute rejection were observed during the first year in the ABOi LDKT group and the ABOc DDKT group (overall 18.4%, p=0.03). Although, no higher incidence of antibody-mediated rejection was observed in the ABOi LDKT group. First year unscheduled readmissions were more frequent in the ABOi group (74.2%) and the DDKT group (74.2%) compared with the ABOc LDKT group (62.7%, p=0.01). Graft function using measured GFR did not differ in any of the groups during the first 5 years (overall 71 ± 25, 63 ±20, 56±25 ml/min/1.73 at 1, 3 and 5 years). In the ABOc LDKT group, 13 (6%) were diagnosed with malignancy during the study period compared with none in the other two groups (p=0.05).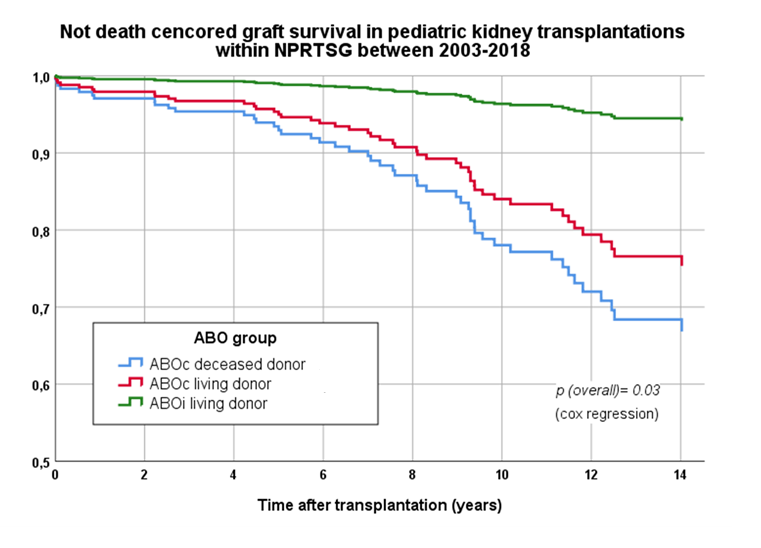
Conclusion: Our findings suggests that pediatric ABOi LDKT is comparable to ABOc LDKT and superior to DD kidney wait listing. We did not observe any significant increase in mortality, graft failure or complications. In fact, graft survival was the highest in the ABOi LDKT group and the incidence of malignancy 0%. ABO-incompatibility should therefore not impede living donation. Finally, if the favorable outcome of ABOi LDKT is ascribed to a protective effect of rituximab induction in pediatric patients calls for further investigation.
Scandiatransplant. Swedish Kidney Foundation. Swedish Order of Freemasons.
