Soufian Meziyerh, Netherlands has been granted the Young Investigator Congress Scientific Award
Real-life tacrolimus levels are associated with biopsy-proven acute rejection after the first year post-kidney transplantation
Soufian Meziyerh1,2, Teun van Gelder3, Danny van der Helm2, Paul J.M. van der Boog1,2, Johan W. de Fijter1,2, Dirk Jan A.R. Moes3, Aiko P.J. de Vries1,2.
1Dept. of Internal Medicine, Division of Nephrology, Leiden University Medical Center, Leiden, Netherlands; 2LUMC Transplant Center, Leiden University Medical Center, Leiden, Netherlands; 3Dept. of Clinical Pharmacy and Toxicology, Leiden University Medical Center, Leiden, Netherlands
Introduction: Evidence to recommend specific target concentrations for immunosuppression beyond the first year of kidney transplantation (KT) is limited. We correlated real-life exposure data of tacrolimus (tac), mycophenolic acid (mpa) with incidence of biopsy-proven acute rejection (BPAR) in kidney transplant recipients (KTRs) in the second and third year after KT.
Methods: For this observational study, we identified 841 KTRs (transplanted from 2005-2018) that were on maintenance therapy with tac, mpa, and prednisolone after year 1 posttransplant. Trough levels (C0) and area-under-the-curve (AUC0-12h) measurements for tac and mpa (performed per protocol and on indication between 6-18 months) were available from 475 KTRs. KTRs were grouped in low and high intra-patient variability (IPV) by the median. Primary outcome was BPAR between year 1-3 posttransplant. Hazard ratios (HR) and confidence intervals (CI) of tac and mpa exposure were adjusted for human leukocyte antigen (HLA)-mismatch and other transplant characteristics.
Results: A total of 16 out of 475 (3.4%) KTRs experienced BPAR between year 1-3 post-transplant. Incidence of BPAR in KTRs with high and low IPV was 5.0% vs 1.3% (p=0.01). Tac was significantly associated with BPAR within year 1-3 posttransplant with an unadjusted HR (uHR) of 0.61 (95%CI: 0.49-0.76; p<0.001) and adjusted HR (aHR) of 0.54 (95%CI: 0.40-0.70; p<0.001) for every 1 µg/L increase in C0, and an uHR of 0.55 (95% CI: 0.42-0.73; p<0.001) and aHR of 0.44 (95% CI: 0.30-0.66; p<0.001) for every 20 µg*h/L increase in AUC0-12h. AUC0-12h-mpa was not associated with BPAR. Probability of BPAR increased exponentially when C0 or AUC of tac was below respectively 5 μg/L or 75µg*h/L (Figure 1). Escalation of Tac levels above 7 μg/L did not lead to meaningful further reduction in BPAR. AUC showed stronger association with BPAR as compared to C0 when using correlation plots (not shown). In KTRs with low IPV lower levels of tac were better tolerated with regards to BPAR as compared to high IPV.
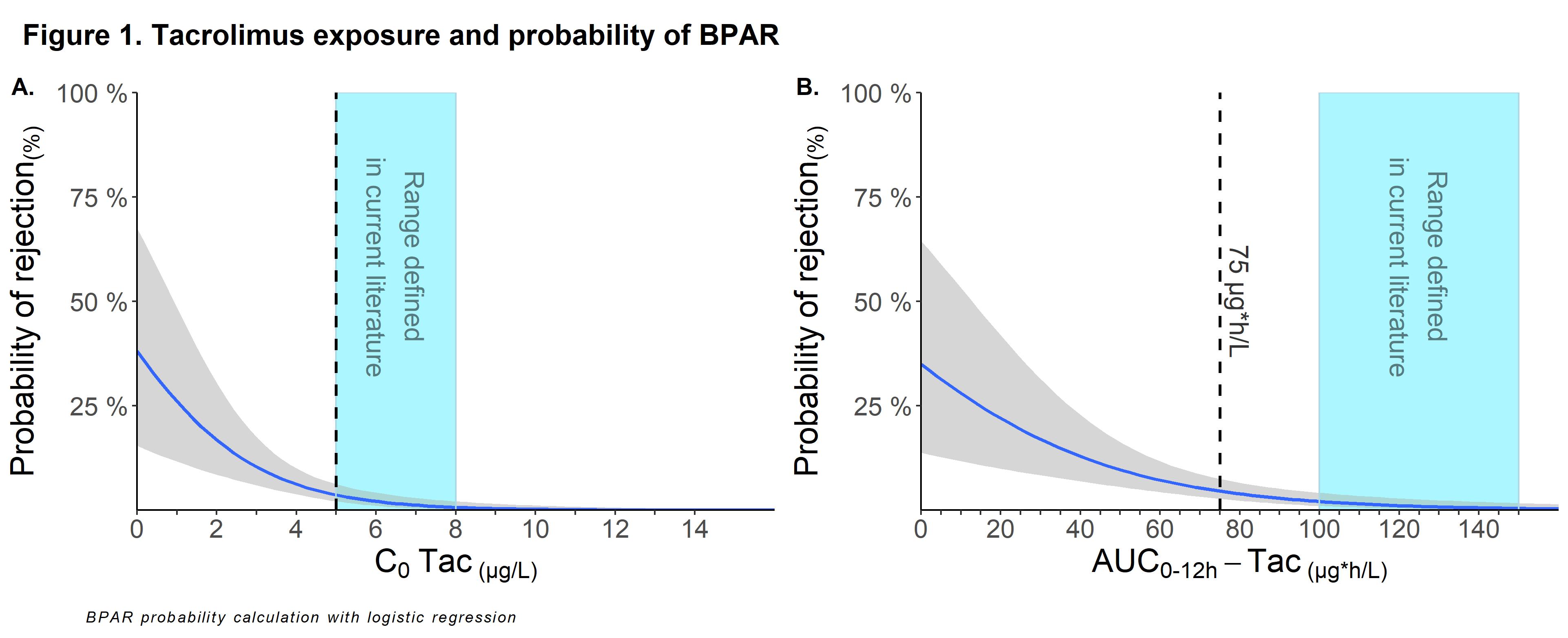
Conclusion: BPAR beyond year 1 posttransplant is uncommon in KTRs on triple therapy with tac C0 > 5 μg/L. We found little evidence to target an (AUC-guided) C0 > 6-7μg/L since a further reduction in the incidence of BPAR between year 1-3 post-transplant was minimal, and risk of toxicity might increase. The association between tac exposure and BPAR was independent of HLA mismatch, MPA exposure and other transplant characteristics. To our knowledge, this is the first study that investigated the impact of tac and mpa exposure beyond the first year posttransplant, which may contribute to better define the therapeutic target window.
