High levels of donor derived cell-free DNA in the early post-transplant phase indicates injury independent of renal function status
Adela Mattiazzi1, Giselle Guerra1.
1Medicine/ Nephrology, University of Miami, Miami, FL, United States
Background: More than 97,000 patients are in the wait list for a kidney transplant in the United States, but only 24,670 transplants were performed in the year 2021. While living donation remained nearly steady in the last 10 years, deceased donation increased 40%. Donation after circulatory death (DCD) donors are a progressively important source of organs. However, DCD donor kidneys have an increased risk of slow (SGF) and delayed graft function (DGF). Patients with DGF often undergo multiple biopsies to differentiate between ischemic acute tubular injury and rejection to prevent early graft loss. Donor derived cell-free DNA (dd-cfDNA) is a non-invasive biomarker for detection of graft injury and rejection in kidney transplant. This study aims to assess the association of dd-cfDNA levels with immediate post-transplant graft function, biopsy proven rejection and rejection treatment in patients with SGF and DGF.
Methods: In this observational single-center study we identified 70 deceased donor kidney transplant recipients at the Miami Transplant Institute with dd-cfDNA testing in the first 30 days after transplantation. Of those, 18 patients had a paired dd-cfDNA and for cause biopsy. Indications for biopsy were creatinine >2ml/dL, donor specific antibodies (DSA), proteinuria, dd-cfDNA levels >1%. Patients were grouped according to allograft function status as DGF, SGF or immediate graft function (IGF). Median dd-cfDNA levels were evaluated in comparison to graft function, biopsy proven rejection, and treatment after rejection, and compared by Kruskal Wallis test between each categorized group. P <0.05 was considered significant.
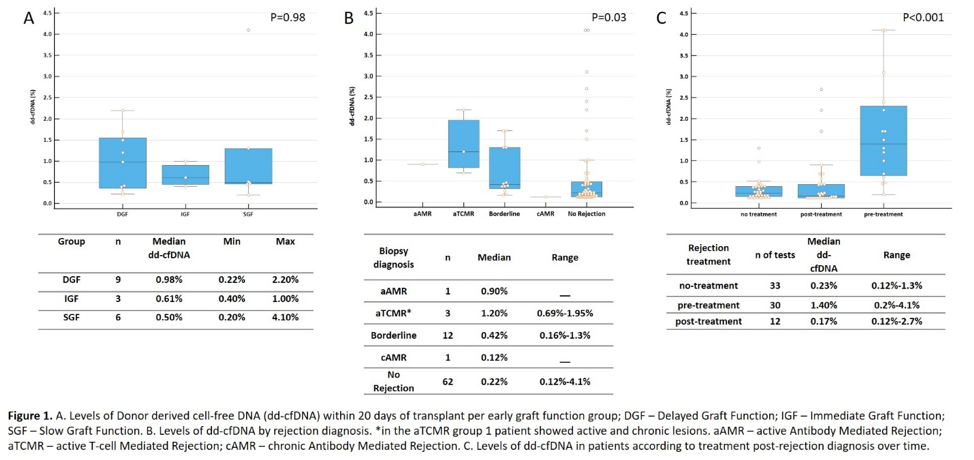
Results: 38.8% of patients received a DCD kidney with a mean KDPI of 58% (±29.8). 27.8% of patients were female. 9 patients had DGF, six had SGF and 3 had IGF. There was no difference in median dd-cfDNA levels between the groups in the first 20 days of transplant Figure 1A. There was no difference in rejection occurrence between the groups. Patients with active rejection had higher levels of dd-cfDNA compared with patients with no rejection and chronic antibody mediated rejection (Figure 1B). When evaluating treatment for rejection, patients that underwent treatment had a significant reduction in dd-cfDNA levels post-treatment (Figure 1C). 7 patients with borderline rejection did not receive treatment, the median dd-cfDNA was 0.37% (range – 0.16-1.3%), while patients with borderline rejection and treatment had 1,3% median dd-cfDNA (range – 0.44-1.7%; p=0.015).
Conclusions: High levels of dd-cfDNA were associated with alloimmune injury irrespective of graft function, showing the clinical utility of this biomarker in the early post-transplant. Patients with DGF are more prone to develop allograft injury, but all patients may benefit of close surveillance in the early post-transplant period. Further evaluation is needed to elucidate the benefits of dd-cfDNA surveillance over biopsy in this clinical setting.