Ischemia minimization improves cardiac function in an ex vivo xeno working heart model
Gannon McGrath1, Franzi Pollok1,2, Anthony Calhoun1, Ryan Chaban1, Zahra Habibabady1, Madelyn Ma1, Kohei Kinoshita1, Margaret Connolly1, Shuhei Miura1, Pratts Shannon1, Lars Burdorf1, Jacob Layer3, Ranjith Anand3, Katherine Hall3, Michele Youd3, Kathryn Stiede3, Wenning Qin3, Mike Curtis3, Richard N Pierson III1.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States; 2Department of Anesthesiology, University of Hamburg-Eppendorf, Hamburg, Germany; 3eGenesis Inc., Cambridge, MA, United States
Introduction: Previous studies showed that ischemia minimization (IM), accomplished by perfusing the heart xenograft during storage, prevents ‘initial cardiac xenograft dysfunction’ (ICXD) and enables prolonged survival following orthotopic cardiac xenotransplantation. Here we report initial observations in a model designed to evaluate effects on heart performance associated with IM and genetic modifications targeted to address xeno-injury mechanisms in a working ex vivo model of ICXD.
Method: Hearts from genetically modified and wildtype (WT) pigs were procured after flushing with cold preservation solution (UW, 4oC) and stored for 3 hours either in cold saline (0.9%, 4oC: cold storage (CS)) or were perfused with oxygenated STEEN Solution with RBCs (IM). IM perfusion at 40 mmHg was initiated at room temperature for 20 minutes to facilitate homogeneous graft perfusion before cooling to 4oC for the remainder of the storage period. The genetically modified hearts either had combined knockouts of three specific xenogeneic carbohydrate genes (GTKO, CMAHKO, b4GALNT2KO: TKO) with variable expression of human transgenes (hTG) including complement and thrombo-regulatory proteins (n=29); or GTKO with additional expression of hCD55 (GTKO.hCD55; n=3). Heart function and laboratory parameters were assessed at specific timepoints on a working heart rig while perfused with freshly collected heparinized whole human blood. Troponin I was used as a marker for myocardial injury.
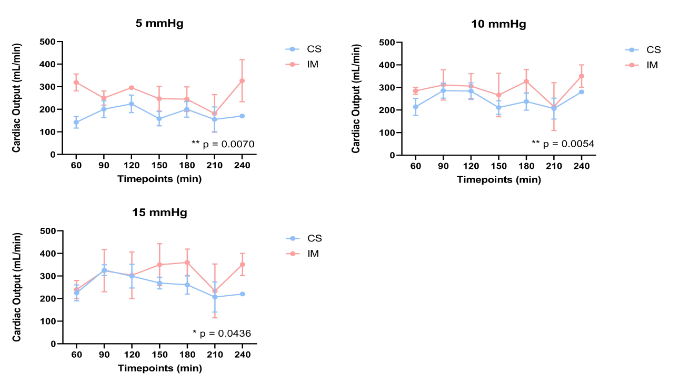
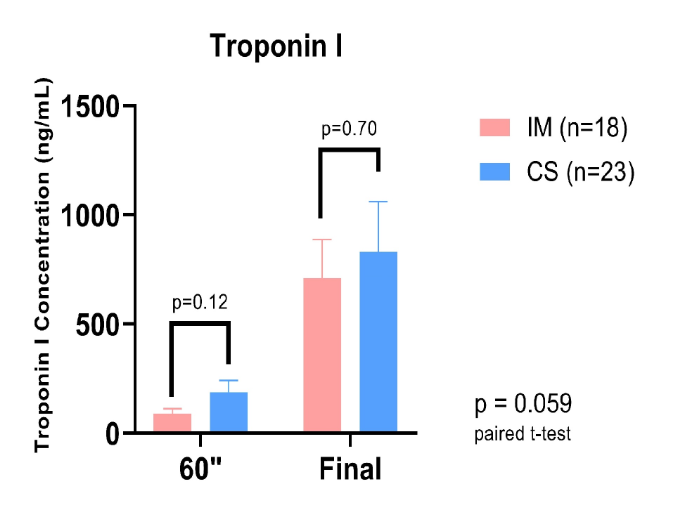
Results: In total, 41 hearts were perfused ex vivo, 23 after CS (TKO + hTG n=14, GTKO.hCD55 n=2, WT n=7), and 18 after IM (TKO + hTG n=15, GTKO.hCD55 n=1, WT n=2). Cardiac output (mL/min) was significantly improved for the IM group at all loading conditions relative to CS, (see figure 1). There was a strong trend toward reduced troponin I release, particularly within the first 60 minutes of perfusion (see figure 2).
Conclusion: IM is protective with respect to cardiac function following pig heart ischemia and subsequent reperfusion with human blood in an ex vivo model of heart xenotransplantation. The hypothesis that variation in physiologic and biochemical outcomes within groups is influenced by variable expression of complement and coagulation pathway regulatory genes is being investigated.