The role of LAG3 in antibody responses to kidney transplantation
Michael Nicosia1, Ran Fan1, Juyeun Lee2, Victoria Gorbacheva1, Ashley Beavers1, Nina Dvorina1, William Baldwin III1, Robert Fairchild1, Booki Min3, Anna Valujskikh1.
1Inflammation and Immunity, Cleveland Clinic, Lerner Research Institute, Cleveland, OH, United States; 2Cardiovascular and Metabolic Sciences, Cleveland Clinic, Lerner Research Institute, Cleveland, OH, United States; 3Department of Microbiology and Immunology, Feinburg School of Medicine, Northwestern Universtiy, Chicago, IL, United States
The functions of coinhibitory receptor lymphocyte activation gene-3 (LAG3) in T cells are well studied, however its role in humoral immune responses remains poorly characterized. The goal of this study was to test the role of recipient LAG3 in a mouse model of renal allograft rejection.
Murine kidney transplant surgeries were performed from C3H (H-2Dk) donors to B6 WT, LAG3-/-, CD4CreLAG3fl/fl & CD19CreLAG3fl/fl recipients (H-2Db) after bilateral nephrectomy. Grafts were evaluated by immunistochemistry. WT recipients were treated with anti-mCD20 or anti-CD8 mAbs to deplete B cells or CD8 T cells, respectively. Immune responses were assessed by flow cytometry, ELISPOT assay, and serum levels of MHC-I and MHC-II-reactive IgG donor specific antibody (DSA) were determined by ELISA.
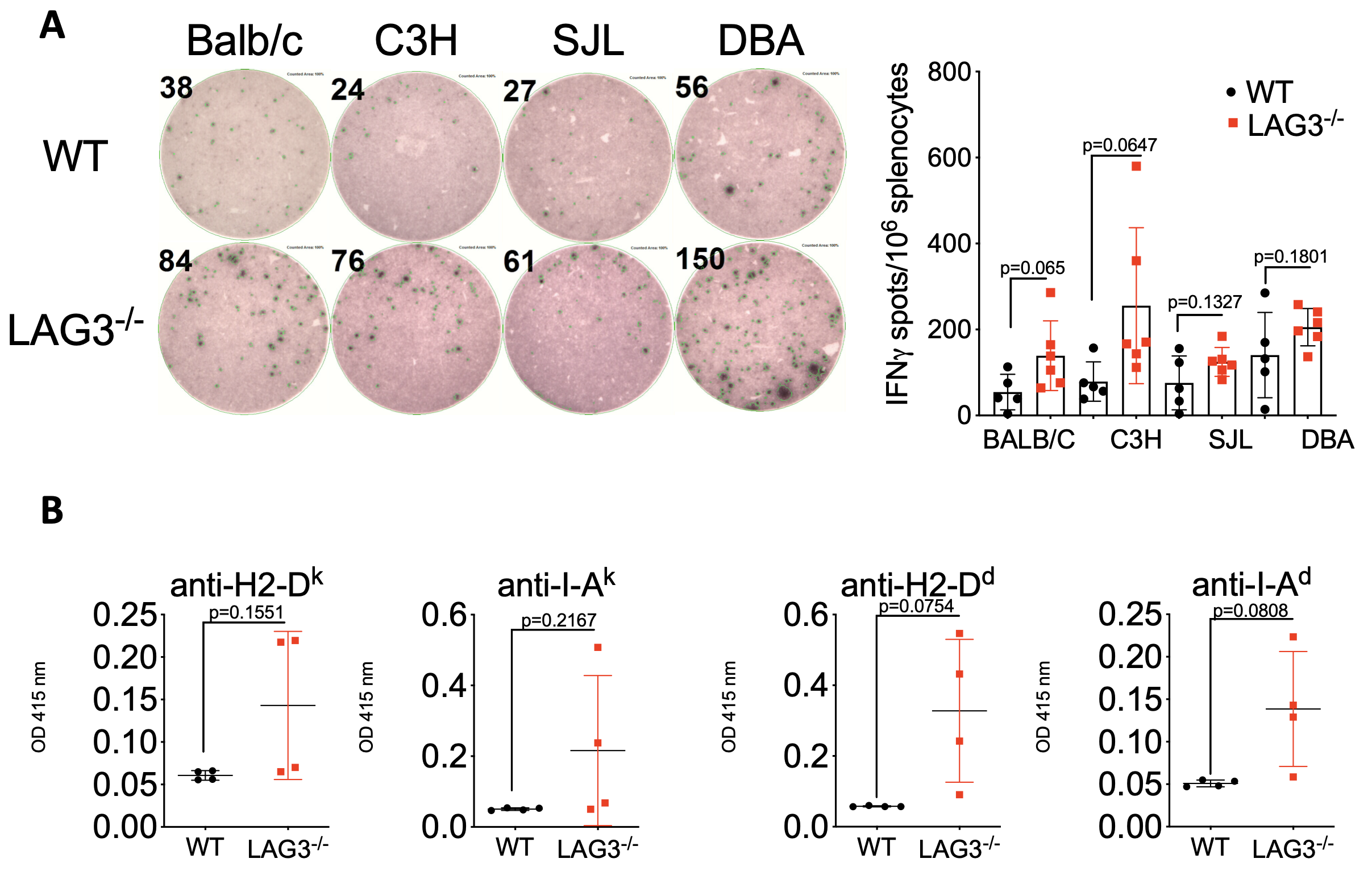
Compared to WT animals, naïve B6.LAG3-/- mice have elevated numbers of CD44hi memory T cells, CXCR5hi follicular T cells, and B220+CD138+ plasma cells, and increased frequencies of memory T cells reactive to H-2Dd, H-2Ds, H-2Dq and H-2Dk (Figure 1A) and increased levels of serum IgG antibodies against H-2Dd, H-2Dk, I-Ad and I-Ak alloantigens (Figure 1B).
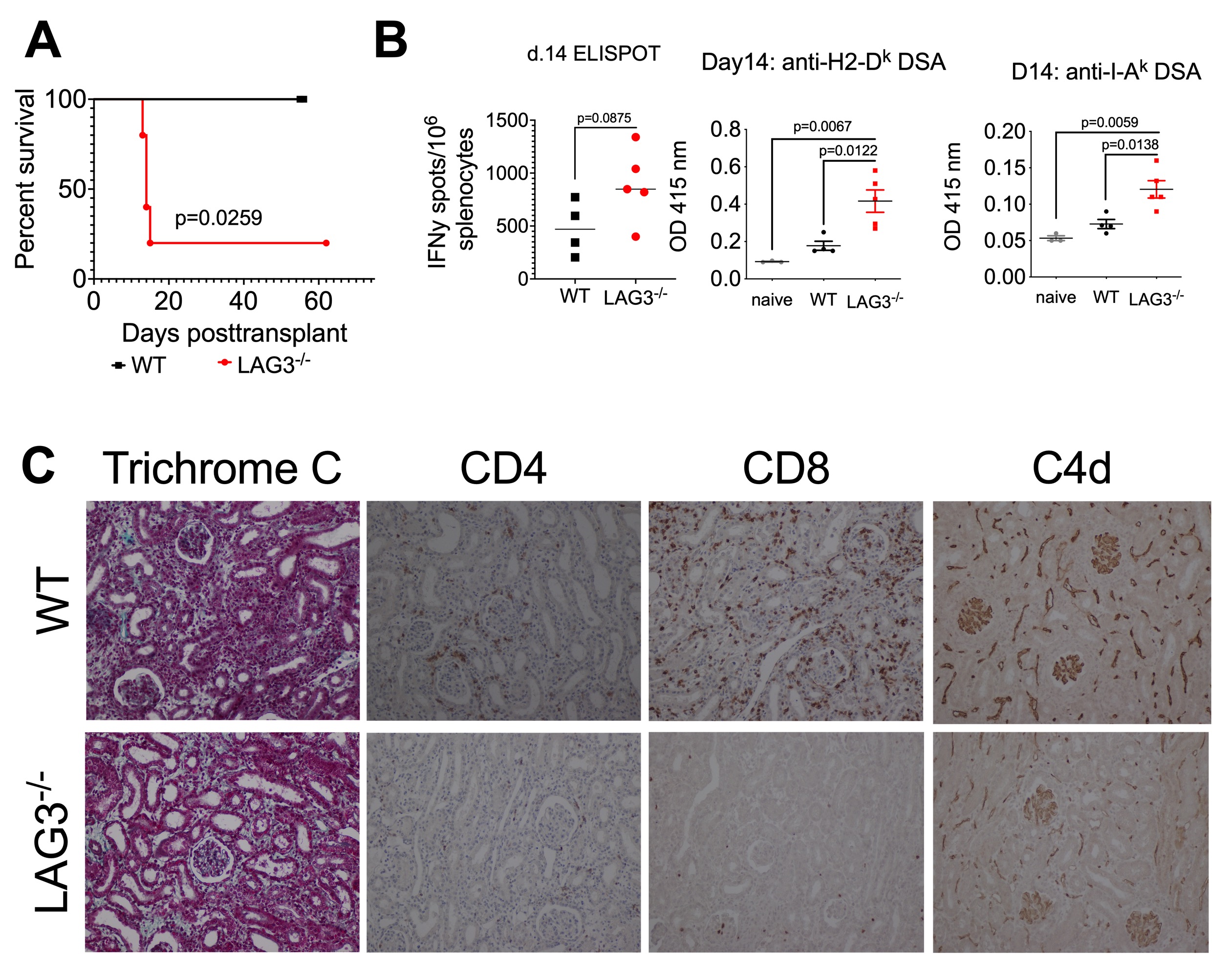
All C3H kidney allografts survived for > 60d (n=5) in WT recipients, whereas recipient LAG3 deficiency led to rapid allograft rejection (MST of 14d, n=5) (Figure 2A) and elevated serum creatinine levels at d14 posttransplant. Compared to WT, LAG3-/- recipients had elevated frequencies of anti-donor IFNγ producing T cells and increased levels of DSA against MHC-I and MHC-II (Figure 2B). Graft histology at rejection revealed minimal T cell infiltration, diffuse C4d staining, atrophic peritubular capillaries, endothelial swelling and edema characteristic of antibody mediated rejection (AMR) (Figure 2C).
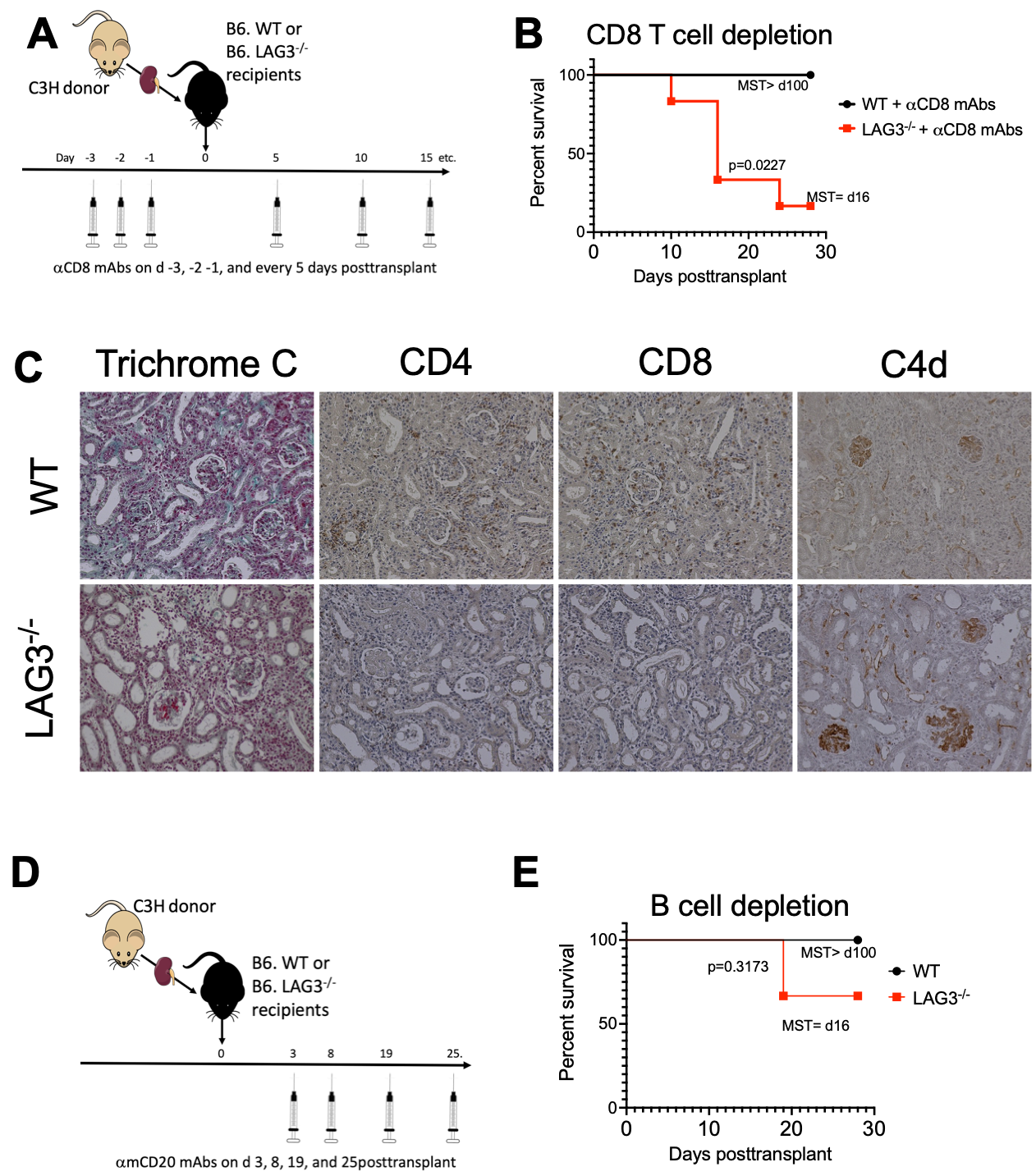
Recipient CD8 T cell depletion did not alter rejection kinetics in LAG3-/- recipients (MST of 16d) (Figure 3A&B), and graft histological findings (Figure 3C) mirrored those of unmanipulated LAG3 deficient recipients (Figure 2C), demonstrating hallmark characterstics of AMR. B cell depletion significantly extended C3H kidney allograft survival (MST of >30d) (Figure 3D&E), suggesting the predominant role of alloantibody rather than T cell mediated rejection in these mice.
However, neither T nor B cell conditional knockout recipients rejected the allograft demonstrating LAG3 expression on both cell types is necessary to mediate rejection.
These findings demonstrate that LAG3 regulates both T and B cell functions in response to kidney allografts, and is an attractive therapeutic target for the prevention of AMR.
