Is incidence of post-transplant diabetes mellitus higher with adoport compared to prograf in patients post renal transplantation?
Sigmund Chan1, Nithya Krishnan1, Ranganatha Rao1.
1Department of Renal Medicine, University Hospital, University Hospitals Coventry & Warwickshire NHS Trust, Coventry, United Kingdom
Introduction: Post-Transplant Diabetes Mellitus (PTDM) refers to those diagnosed with diabetes mellitus after receiving a solid organ transplant. PTDM in the renal transplant patient has been associated with adverse cardiovascular disease outcomes, higher rates of graft rejection and infection. Current literature has reported the incidence of PTDM ranges from 9% to 39% within the first year post transplant. Commonly used immunosuppressants e.g. Tacrolimus can increase risk of PTDM. In recent years, many regional renal centres in the United Kingdom (UK) have changed Tacrolimus formulations from Prograf® (Brand) to Adoport® (Generic). This was mainly due to cost saving implications. At our local adult renal transplant centre in the UK, we have anecdotally speculated a higher incidence of PTDM between the two formulations. Thus, we have reviewed our incidence of PTDM in both groups.
Method: Data was obtained from our centre's patient database on all adult patients who received a kidney transplant (living & deceased donor) between 2013 to 2019. Our criteria for diagnosing PTDM was in concordance with international recommendations.
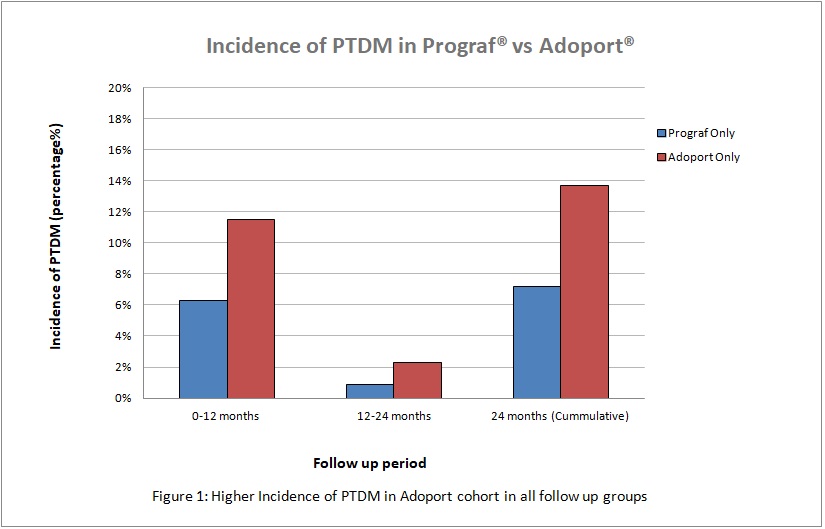
Incidence of PTDM was reviewed for the following cohorts: those patients on Prograf® only versus Adoport® only. Incidence was reviewed within 12 months, 12-24 months and 24 months post transplant. Fisher exact statistical analysis was used.
Results: Between 2013-2019, 452 patients received a renal transplant at our centre. 204 patients were started on Prograf®, 213 on Adoport®. The remainder were not on either medication. After further exclusion criteria, a total of 198 patients were identified with 24 months follow up post transplant (111 in Prograf® cohort, 87 in Adoport® cohort).
At 12 months follow up post-transplant, the overall incidence of PTDM in both cohorts was 8.6% (17/198). Adoport® cohort (10/87) had a higher incidence of PTDM compared to Prograf® (7/111), {11.5% versus 6.3% p=0.21}.
Between 12-24 months post transplant, two further patients on Adoport® developed PTDM compared to one further patient in Prograf® cohort.
Cumulatively, over a 24 months period, our study showed an overall incidence of PTDM in both cohorts of 10.1% (20/198). Similarly, there was a higher incidence of PTDM in the Adoport® group (12/87) compared to Prograf® (8/111), {13.7% versus 7.2%, p=0.16). Average time to diagnosis of PTDM was 6.9months (Prograf®) compared to 7.0months (Adoport®).
Conclusion: At present, we are the only study in medical literature to compare different formulations of Tacrolimus (Adoport® and Prograf®) and their incidence of PTDM in renal transplant patients. Our data suggested a higher incidence of PTDM in those renal transplant patients who take Adoport® compared to Prograf®. Although our findings were not statistically significant, likely due to small sample size, further research into the different preparations of Tacrolimus' risk of developing PTDM is warranted.