Case report: nephrotic-range proteinuria in a deceased donor recipient with acute antibody mediated rejection and familiar FSGS
Claudia CC Albuquerque1, Daniel N Gazolla1, Jose Otto Reusing Junior1.
1Kidney Transplant Service, Hospital das Clínicas, University of São Paulo School of Medicine, São Paulo, Brazil
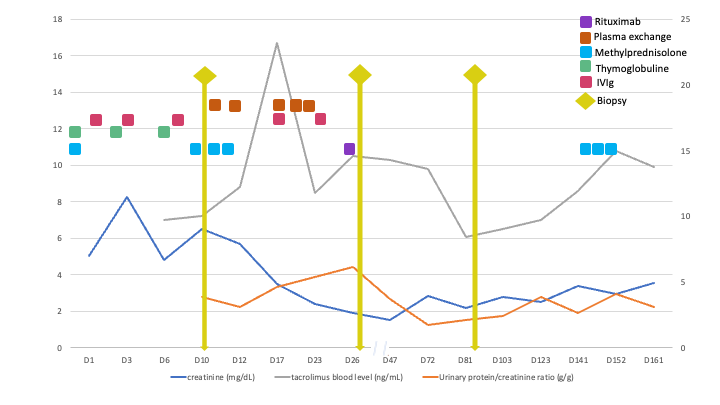
A 47yo female patient presented early after transplant a nephrotic-range proteinuria despite treatment for active antibody mediated rejection (AMR). She had undergone a deceased donor kidney transplantation after a desensitization protocol with immunoglobulin for HLA antibodies; immunosuppression consisted of 6 mg/kg thymoglobulin for induction and triple therapy (mycophenolate, tacrolimus, prednisone) for maintenance. She was previously diagnosed with familiar focal and segmental glomerulosclerosis (FSGS) due to a IVS9+5G>A mutation of the WT1 gene; her clinical history was characterized by corticorresistent nephrotic syndrome and arterial hypertension that evolved to anuric end-stage kidney disease and 16y of hemodialysis treatment. Her daughter was also affected by FSGS related to WT1 mutation. The caucasian female donor of standard criteria had known systemic hypertension and final creatinine of 0.89 mg/dl; previous A68 donor-specific antibody (DSA) was absent at Tx. Graft dysfunction and a macro-proteinuria were present since the first week. Day 10 graft biopsy displayed AMR features and C3 mesangial deposition; resurgence of A68 DSA and positive flow-cytometry crossmatch confirmed AMR which was treated with pulse corticosteroid, plasmapheresis, human immunoglobulin and rituximab. A second biopsy revealed collapsing glomerulopathy with C3 deposition, C4d negative, and minimal microvascular inflammation. Because the early FSGS features should not be the recurrence of the familiar FSGS nor come from the rejection episode, a donor cause was pursued. The other kidney recipient from the same donor also developed early proteinuria and graft dysfunction, but no rejection. Sanger sequencing of donor APOL1 gene revealed G1/G2 risk alleles. Final diagnosis was APOL1 nephropathy (collapsing glom.) from the donor with superimposed AMR. This case illustrates the unexpected proteinuria from the initial diagnosis of rejection and familiar FSGS.