
Differential immunogenicity of mRNA-1273 versus BNT162b2 as a third vaccine dose for solid organ transplant recipients seronegative after two BNT162b2 doses
Amy Chang1, Teresa PY Chiang1, Jennifer L Alejo1, Jonathan Mitchell1, Jake D Kim1, Aura T Abedon1, Robin K Avery2, Aaron AR Tobian2, Allan B Massie3, Macey L Levan3, Daniel S Warren1, Jacqueline M Garonzik-Wang4, Dorry L Segev3, William A Werbel2.
1Surgery, Johns Hopkins School of Medicine, Baltimore, MD, United States; 2Medicine, Johns Hopkins School of Medicine, Baltimore, MD, United States; 3Surgery, New York University Grossman School of Medicine, New York, NY, United States; 4Surgery, University of Wisconsin School of Medicine & Public Health, Madison, WI, United States
Introduction: There is no consensus as to the optimal SARS-CoV-2 vaccination sequence in solid organ transplant recipients (SOTRs). Anti-spike antibody response to the two-dose BNT162b2 mRNA vaccine series is poor in SOTRs, and it is possible that the higher-dose mRNA-1273 vaccine could improve immunogenicity in suboptimal responders, who are at high risk for SARS-CoV-2 infection.
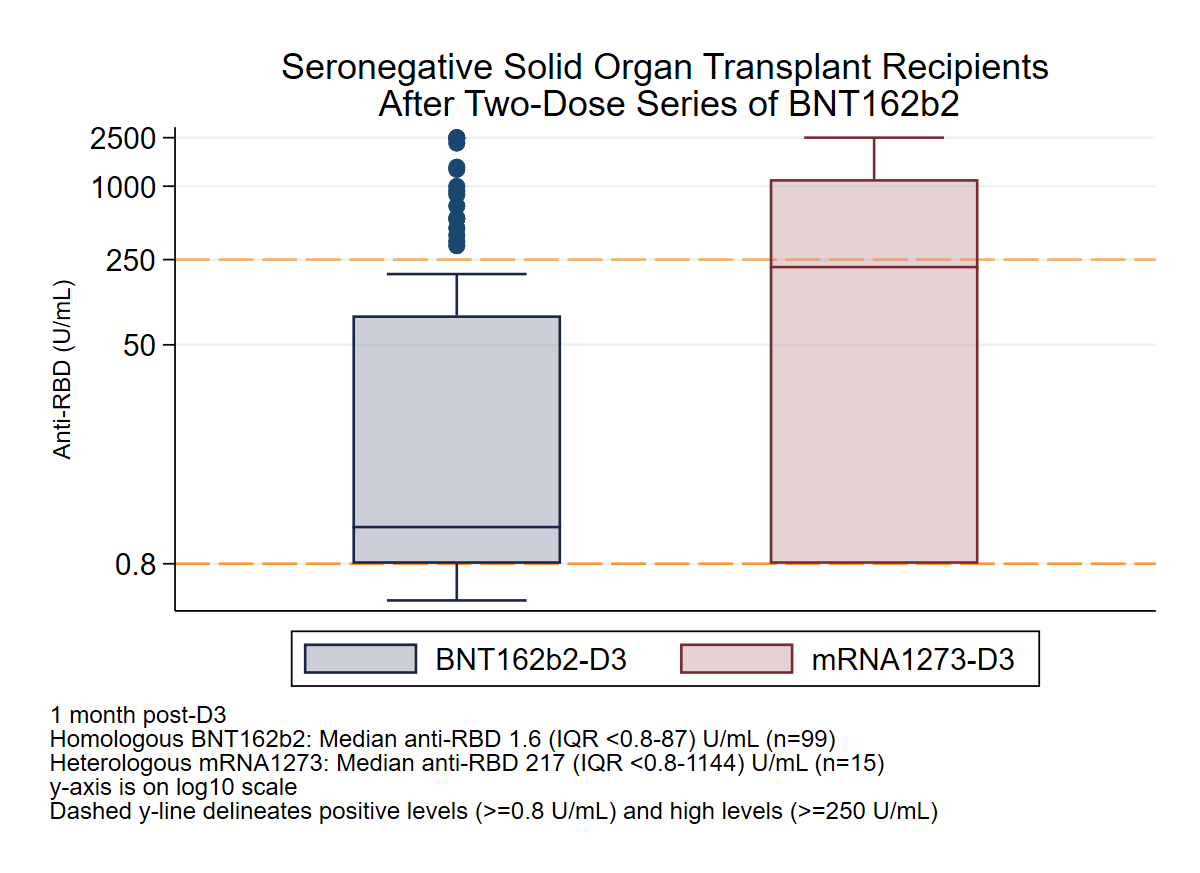
Methods: We evaluated 114 SOTRs who tested negative on an anti-spike antibody assay after two doses of BNT162b2 and had a third dose (D3) of either homologous BNT162b2 or heterologous mRNA-1273 vaccine. The proportion of seroconversion [WW1] at 1 month post-D3 was compared between groups using Fisher's exact testing. Additionally, the rate of high-titer response at 1 month post-D3, defined as achieving an antibody level associated with neutralizing capacity versus the ancestral SARS-CoV-2 variant (≥250 binding antibody units), was analyzed using multivariable Poisson regression with robust standard error, adjusting for mycophenolate (MMF) use, age, and years since transplant.
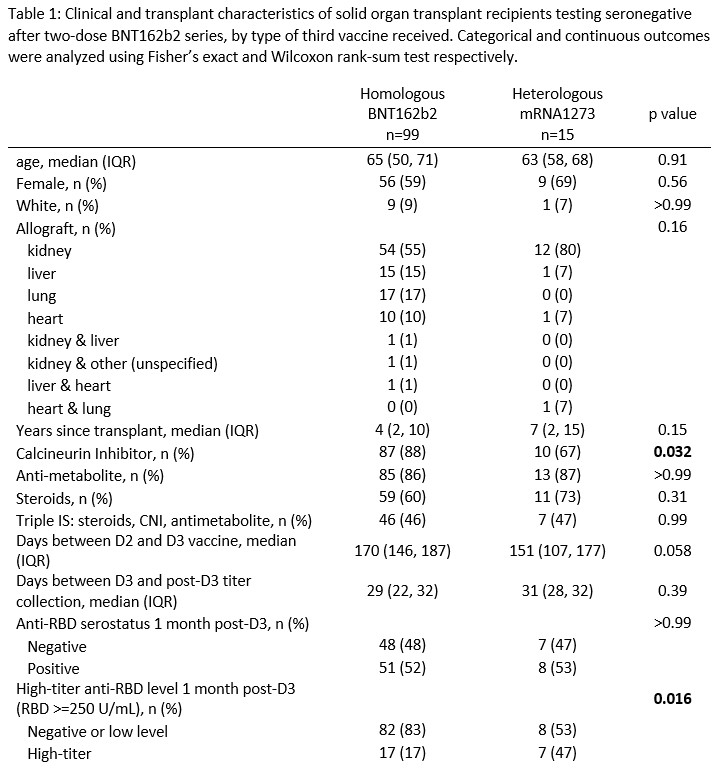
Results: Overall, 99 SOTRs received homologous BNT162b2 D3 and 15 received heterologous mRNA-1273 D3. There was no significant difference in age (median 65 [IQR 50-71] vs 63 [58-68] years), sex (59% vs 69% female), time since transplant (median 4 [IQR 2-10] vs 7 [2-15] years), or MMF use (84% vs 80%) between BNT162b2 and mRNA-1273 D3 groups, though calcineurin inhibitor use differed (88% vs 67%, p=0.03). Seroconversion at 1 month post-D3 was similar between BNT162b2 and mRNA-1273 D3 groups (52% vs 53%, p>0.99). However, the mRNA-1273 D3 group was significantly more likely to develop high-titer response at 1 month post-D3 (17% vs 47%, p=0.02). After adjusting for demographic and transplant characteristics, the mRNA-1273 D3 group was 2.71-fold more likely to develop high-titer response at 1 month post D3 (incidence rate ratio [95% CI] 2.71 [1.30-5.64], p=0.008).
Conclusion: In SOTRs without initial response to two-dose BNT162b2 vaccination, a heterologous mRNA-1273 D3 showed similar seroconversion rates (~50%) to homologous BNT162b2 D3, but was significantly more likely to generate high-titer responses. Further directions include assessment of COVID-19 breakthroughs among vaccine regimens, as well as exploring the neutralizing capacity and durability of antibody response to heterologous platforms.
ASTS Jon Fryer Resident Scientist Award. National Institute of Diabetes and Digestive and Kidney Diseases: T32DK007732 (Dr. Chang), T32DK007713 (Dr. Alejo), K01DK101677 (Dr. Massie), K01DK114388-03 (Dr. Levan), and K23DK115908 (Dr. Garonzik-Wang). National Institute of Allergy and Infectious Disease: K23AI157893 and U01AI138897 (Dr. Werbel) and K24AI144954 (Dr. Segev).

right-click to download
