
SARS-CoV-2 antibody response to a third dose of homologous mRNA vaccination in liver transplant recipients
Amy Chang1, Alexandra T Strauss2, Jennifer L Alejo1, Teresa PY Chiang1, Nicole F Hernandez1, Laura B Zeiser1, Brian J Boyarsky1, Robin K Avery2, Aaron AR Tobian2, Macey L Levan4, Daniel S Warren1, Allan B Massie4, Jacqueline M Garonzik-Wang3, Dorry L Segev4, William A Werbel2.
1Surgery, Johns Hopkins School of Medicine, Baltimore, MD, United States; 2Medicine, Johns Hopkins School of Medicine, Baltimore, MD, United States; 3Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States; 4Surgery, New York University Grossman School of Medicine, New York, NY, United States
Purpose: Liver transplant (LT) recipients have a decreased response to 2 doses of SARS-CoV-2 vaccine compared to the general population, so we aimed to understand the response to a third dose to inform vaccination strategies.
Methods: LT recipients with 3 congruent mRNA vaccines and antibody levels pre- and post-dose 3 (D3) were included. Those who reported a prior COVID-19 diagnosis or used belatacept were excluded. The latest anti-spike antibody level collected between the second (D2) and third dose (D3) was compared to the antibody level at 1 month post-D3. Samples were tested with Roche Elecsys Anti-Sars-CoV-2 enzyme immunoassay (EIA) (positive ≥0.8 U/mL) or EUROIMMUN EIA (positive ≥1.1 AU).
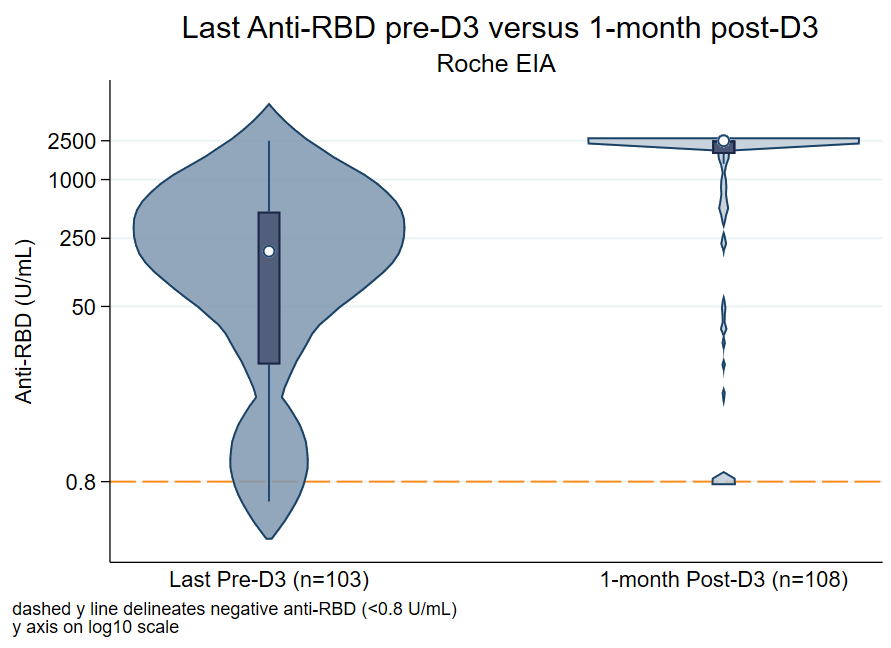
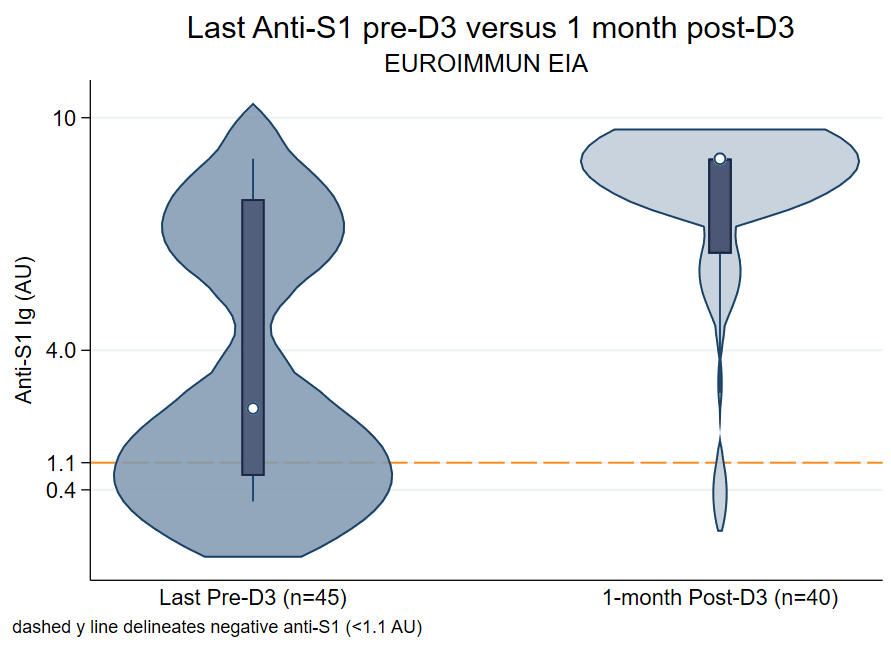
Results: 149 participants completed 3 congruent doses of BNT162b2 (54%) or mRNA-1273 (46%) vaccines between 5/15/2021 - 11/20/2021. The median (IQR) time of latest pre-D3 antibody collection was 119 (92, 179) days post-D2. The median time of 1-month post-D3 antibody collection was 30(26, 33) days. The median time between D2 and D3 was 168 (149-188) days. Overall, 116/149 (78%) had a seropositive response pre-D3, and 141/149 (95%) were positive post-D3 (Figure 1). Among the 116 seropositive pre-D3, 116/116 (100%) remained seropositive post-D3. Among the 33 seronegative pre-D3, 25/33 (76%) converted to seropositive post-D3. Risk factors that were statistically significantly associated with negative post-D3 were mycophenolate mofetil use (75% vs 27%, p=0.009), BNT162b2 series (100% vs 52%, p=0.008), and seronegativity pre-D3 (100% vs 18%, p<0.001).
Conclusion: LT recipients that were positive or negative pre-D3 had an excellent response to a third dose of mRNA vaccine, which is beyond what has been shown in other solid organ transplant recipients. Investigation in immunogenicity remains essential to protect vulnerable groups and guide policy.
Figure: Antibody levels of latest pre-D3 versus 1 month post-D3, categorized by assay performed. The median (IQR) anti-RBD levels increased from pre to post-D3 by 12.9-fold (194 U/mL to 2500 U/mL), and median (IQR) anti-S1 levels increased by 4.5-fold (1.99 AU to 8.94 AU). The reference line delineates negative levels for anti-RBD (<0.8 U/mL) and anti-S1 (<1.1 AU) EIA.
National Institute of Diabetes and Digestive and Kidney Diseases: T32DK007732 (Dr. Chang), T32DK007713 (Dr. Alejo), F32DK124941 (Dr. Boyarsky), K01DK114388 (Dr. Levan), K01DK101677 (Dr. Massie), and K23DK115908 (Dr. Garonzik-Wang). Johns Hopkins Pearl M. Stetler Research Fund. National Institute of Allergy and Infectious Disease: K23AI157893, U01AI138897-S04 (Dr. Werbel), and K24AI144954,U01AI138897 (Dr. Segev) . Ben-Dov family. Trokhan Patterson family.

right-click to download
