MRNA based vaccines against SARS-CoV-2 do not alter cPRA in patients awaiting kidney transplant
Mike Rewinski2,3, Pamela Doyle2,3, Daniel Durkin2,3, Sandy Vicki2,3, Joseph Devivo2,3, Roger Caruk2,3, Andrew Haberman3, Debera Palmeri3, Pamela Cyr3, Glyn Morgan1,3,4, Oscar Serrano1,3,4, Bishoy Emmanuel1,3,4, Zeynep Ebcioglu3,5, Joseph U Singh3,5, Rebecca Kent3,5, Xiaoyi Ye3,5, Joseph Tremaglio3,5, Laurine Bow2,3,6, Wasim Dar1,3,4.
1Surgery, Hartford Hospital, Hartford, CT, United States; 2HLA Lab, Hartford Hospital, Hartford, CT, United States; 3Transplant and Comprehensive Liver Center, Hartford Hospital, Hartford, CT, United States; 4Surgery, University of Connecticut School of Medicine, Farmington, CT, United States; 5Medicine, University of Connecticut School of Medicine, Farmington, CT, United States; 6Surgery, Yale University School of Medicine, New Haven, CT, United States
Introduction: A recent case report in a patient awaiting living donor kidney transplant demonstrated vaccination against SARS CoV-2 with resulted in B-cell activation causing emergence of DSA. This raises the questions as to whether vaccination against SARS CoV-2 should be considered a sensitizing event and warrant increased testing of sera for anti-HLA antibodies in patients awaiting kidney transplant.
Methods: We sought to anwer this question by reviewing anti-HLA antibody testing results in sensitized and unsensitized patients before and after vaccination against SARS CoV-2. Patients were selected on the basis of having received at least two doses of either the Pfizer or Moderna SARS CoV-2 vaccine and have sera tested before and after receiving the vaccinations. 12 sensitized and 10 unsensitized who met criteria were indentified. Sera was tested using Luminex single antigen bead platforms per protocol. Results included anti-HLA antibody specificity as well as MFI ranges. cPRA was calculated from these values.
Results: In 11/12 sensitized patients vaccination against SARS CoV-2 did not result in production of new anti-HLA antibodies nor appreciaciably change the MFI of exisiting antibodies. One sensitized patient did have an increased number of both class I and class II antibodies after vaccination but this patient also stopped immunsuppression prior to receiving the vaccine. Thus this patient's results are likely attributable to alterations in immunosuppression and not from vaccination (Figure 1). In unsensitized patients, there was no de novo development of anti-HLA antibodies after vaccination.
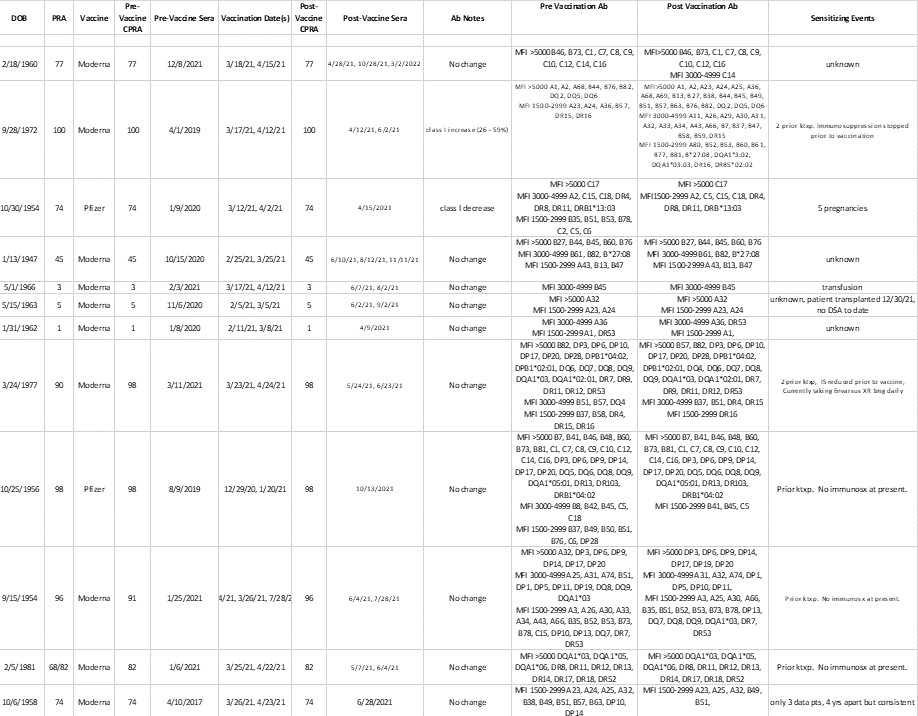
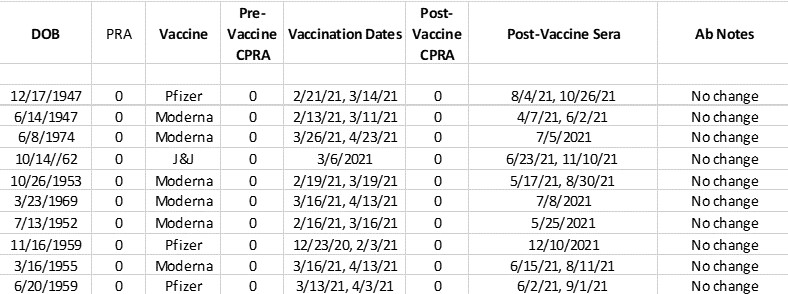
Conclusion: Vaccination against SARS CoV-2 did not result in de novo development of anti-HLA antibodies in unsensitized patients and did not alter the specificity nor the MFI's of existing anti-HLA antibodies in sensitized patients. This indicates that there is no braod need for increased testing of sera in patients awaiting kidney transplant after SARS CoV-2 vaccination.

right-click to download
