Transplantation of organs from SARS-CoV-2-positive donors to uninfected (non-lung) solid organ transplant recipients has not resulted in significant SARS-CoV-2 related morbidity or mortality
Wasim Dar1,2,4, Jason Wade1, Ayyaz Ali2,3,4, Joseph A. Radojevic2,3,5, Michael T. Lawlor2,3,5, Jonathan A. Hammond2,3,4, Jason Gluck2,3,5, Andrew D. Feingold2,3,5, Abhishek Jaiswal2,3,5, Zeynep Ebcioglu2,5, Michael Einstein2,5, Glyn Morgan1,2,4, Bishoy Emmanuel1,2,4, Xiaoyi Ye2,5, Joseph Singh2,5, Eva U. Sotil2,5, Colin Swales2,5, Rebecca Kent2,5, Elizabeth Richardson2,5, Joseph Tremaglio2,5, Faiqa Cheema2,5, Oscar Serrano1,2,5.
1Surgery, Hartford Hospital, Hartford, CT, United States; 2Transplant and Comprehensive Liver Center, Hartford Hospital, Hartford, CT, United States; 3Center for Advanced Heart Failure and Pulmonary Vascular Disease, Hartford Hospital, Hartford, CT, United States; 4Surgery, University of Connecticut School of Medicine, Farmington, CT, United States; 5Medicine, University of Connecticut School of Medicine, Farmington, CT, United States
Introduction: The SARS-CoV-2 pandemic has conferred significant morbidity and mortality across the globe, especially for solid organ transplant (SOT) recipients. This has led to reluctance of using organs from SARS-CoV-2 infected donors due to concerns of disease transmission. However, this must be offset with an increase in wait list mortality. We sought to evaluate the use of organs from SARS-CoV-2 donors into (non-Lung) Solid Organ Transplant Recipients in a prospective fashion.
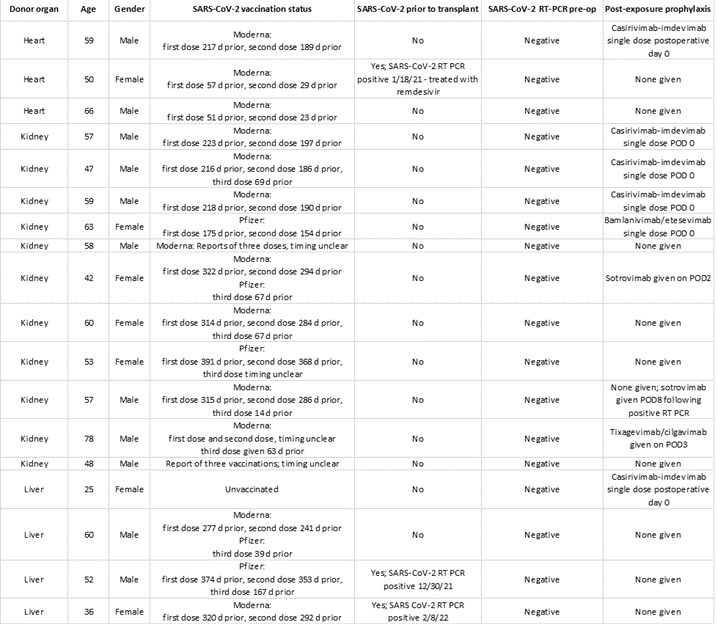
Methods: We evaluated the use of organs from SARS-CoV-2 donors between 6/17/2021 to 2/24/2022. 18 patients were transplanted with SARS-CoV-2-positive donor (COVID+) organs: 3 heart, 4 liver, and 11 kidney recipients. SOT recipients were tested for presence of SARS-CoV-2 by RT-PCR prior to and after their operations. Induction immunosuppression was standard and not altered from protocol. Postoperatively, recipients were kept in modified droplet precautions and received post-exposure treatment recommended by Transplant Infectious Disease. Treatment regimens included remdesivir and monoclonal antibodies directed against SARS-CoV-2 (Figure 1). Perioperative and post discharge care was administered by a multidisciplinary care team with no significant alterations in care protocols.
Results: SOT recipients of COVID+ organs at our institution have not experienced any significant morbidity or mortality from SARS-CoV-2. All patients are alive at current follow up with excellent allograft function with a median follow up for heart, liver, and kidney recipients of 235, 67, and 86 days respectively. All patients were vaccinated except for one liver patient who was status 1A due to fulminant acute liver failure. 13/18 patients had post-operative nasopharyngeal swabbing for RT-PCR based viral testing and two tested positive for SARS-CoV-2 at POD #8. Neither patient experience clinical symptoms of disease. Use of of SARS-CoV-2 did not appear to increase inpatient hospital stay. Median LOS for heart, liver, and kidney recipients was 40, 12.3, and 7.4 days respectively. Neither pre-transplant vaccination, use of COVID+ organs, nor post exposure prophylaxis resulted in increased risk of immunologic complications as only one recipient (heart) has experienced an acute cellular rejection episode to date
Conclusion: Our experience indicates that COVID+ organs can be utilized successfully. The decision to use these organs was based on organ quality, individual patient need at the time the organ offer was made and whether a potential similar quality, COVID negative organ would be available to the patient in the near future. As evidenced by our data, successful use of COVID+ organs is dependent on multidisciplinary wait list and perioperative management to ensure appropriate donor:recipient selection and mitigation of transmission risk with use of post-exposure prophylaxis.

right-click to download
