Humoral response to SARS-COV-2 vaccination in kidney transplant recipients
Maria Butiu1, Bogdan Obrisca2, Lena Sibulesky3, Ramasamy Bakthavatsalam3, Kelly D. Smith4, Idoia Gimferrer5, Gener Ismail2, Nicolae Leca1.
1Division of Nephrology, University of Washington, Seattle, WA, United States; 2Department of Nephrology, Fundeni Clinical Institute, Bucharest, Romania; 3Division of Transplant Surgery, University of Washington, Seattle, WA, United States; 4Department of Laboratory Medicine and Pathology, University of Washington, Seattle, WA, United States; 5Immunogenetics/HLA Laboratory, Bloodworks Northwest, Seattle, WA, United States
Introduction: We sought to evaluate the humoral response to SARS-CoV-2 vaccination in a cohort of kidney transplant (KT) recipients and to identify factors associated with poor humoral immune response.
Method: Serum samples collected from COVID-19 vaccinated kidney transplant (KT) recipients from 3/1/21-4/26/21 were tested for COVID-19 antibodies using a novel multi-antigen detection Luminex platform (BioRad). We measured the specific anti-SARS-CoV-2 IgG antibodies against the individual components of the trimeric spike protein, namely spike 1 (S1), spike 2 (S2) and receptor-binding domains (RBDs). In addition, documentation of previous infection was assessed by measuring anti-nucleocapsid antibodies.
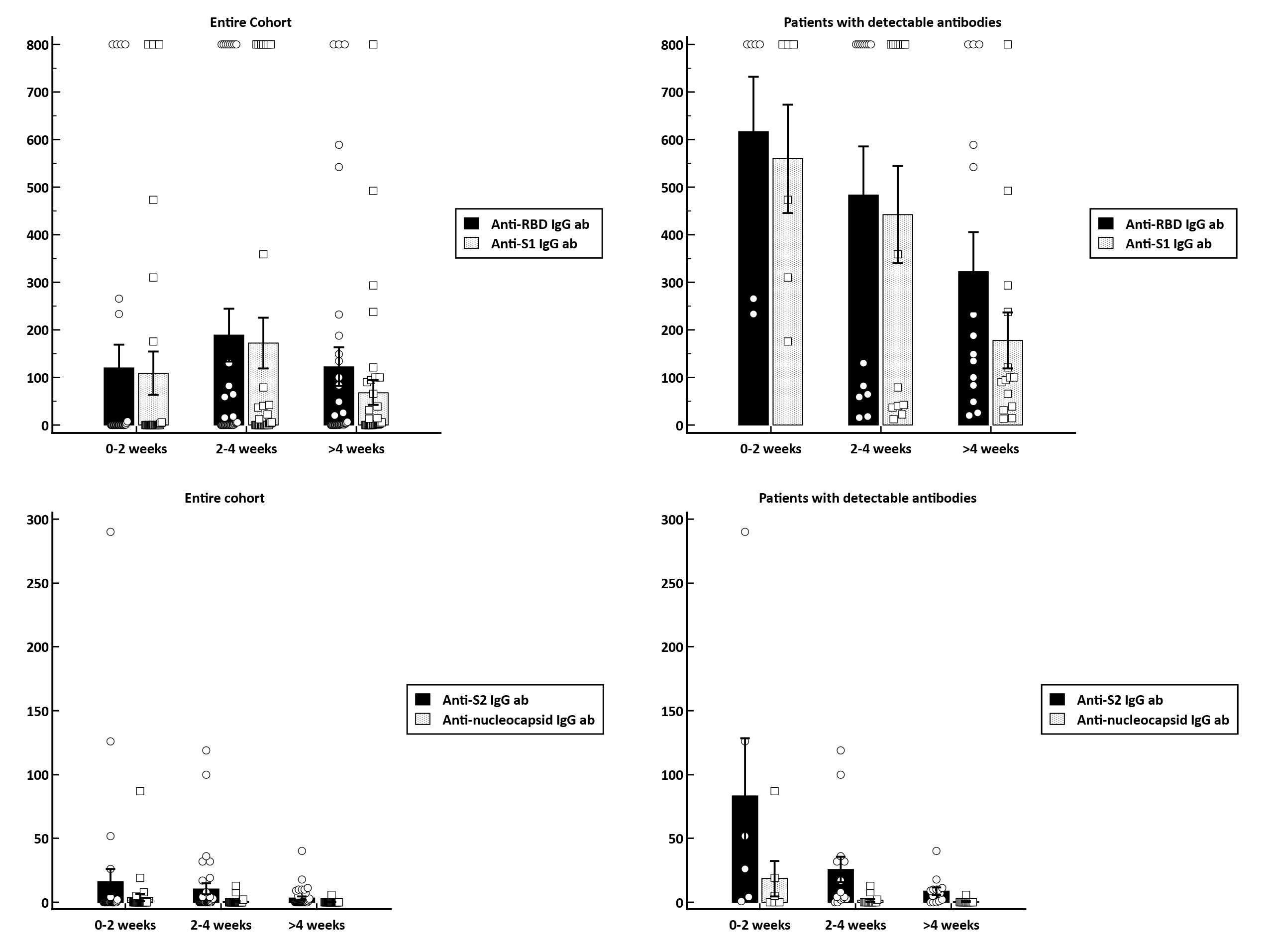
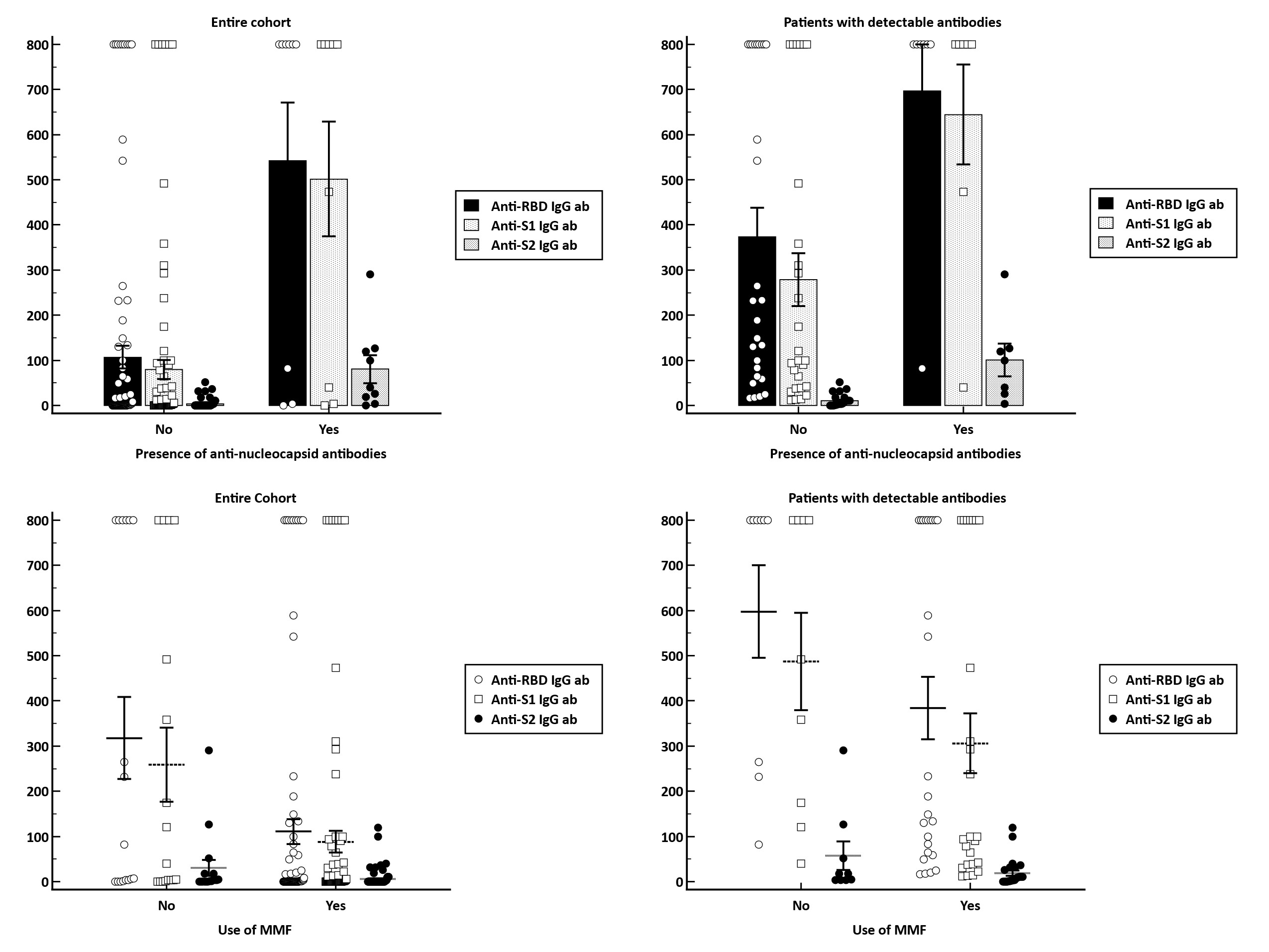
Results: The study cohort enrolled 104 KT recipients that underwent assessment of serological responses at a median 3 weeks (IQR: 1.6-4.9) after vaccination. The majority of patients received either the BNT162b2 (50%) or mRNA-1273 (47.1%), with only 2.9% of patients receiving the Ad26.COV2.S vaccine. Overall, 32.7% of patients became seropositive with a median anti-S1 IgG and anti-RBD IgG levels of 403 BAU/ml (IQR:82-800) and 206 BAU/ml (IQR:41-800). In terms of predictive factors for vaccine response, we identified that those on an immunosuppressive regimen containing MMF were less likely to develop a seroconversion (relative risk [RR], 0.54 [95% CI, 0.31-0.94]; p=0.03). By contrast, patients with evidence of previous infection as documented by anti-nucleocapsid positivity were significantly more likely to became seropositive (RR, 2.73; 95%CI, 1.7-4.39; p<0.001). Regarding the temporal evolution of humoral response, there is a clear tendency for a weaning of antibody response over time. In patients with a vaccine response, the median titer of anti-RBD antibodies decreased from 800 BAU/ml (IQR:257-800), in patients with serum samples within 2 weeks after vaccination, to 168 BAU/ml (IQR:74-641), in patients with samples taken after more than 4 weeks post-vaccination (p=0.15). Similarly, the anti-S1 IgG antibody titers were higher in patients with serum samples taken early after vaccination [636 BAU/ml (IQR:276-800), for 0-2 weeks; 579 BAU/ml (IQR:39-800), for 2-4 weeks and 97 BAU/ml (IQR:37-251) for > 4 weeks; p=0.05]. In multivariate logistic regression analysis, we identified the presence of anti-nucleocapsid IgG antibodies as being independently associated with approximately 8-fold higher chance for a seroconversion (HR, 7.96; 95%CI, 1.26-50.1), while use of MMF-containing immunosuppressive regimens decreased the chance of humoral response by approximately 66% (HR, 0.34; 95%CI, 0.1-1.11).
Conclusion: Kidney transplant recipients have a poor humoral immune response to a two-dose regimen of SARS-CoV-2 vaccine. Previous natural infection increase the likelihood for development a seroconversion, while MMF-containing regimens decreased vaccine responsiveness.

right-click to download
