Elevated donor-derived cell-free DNA associates with subsequent decline of allograft function
Bogdan Obrisca1, Maria Butiu2, Lena Sibulesky3, Ramasamy Bakthavatsalam3, Kelly D. Smith4, Idoia Gimferrer5, Gener Ismail1, Nicolae Leca2.
1Nephrology, Fundeni Clinical Institute, Bucharest, Romania; 2Nephrology , University of Washington, Seattle, WA, United States; 3Transplant Surgery, University of Washington, Seattle, WA, United States; 4Laboratory Medicine and Pathology, University of Washington, Seattle, WA, United States; 5Immunogenetics/HLA Laboratory, Bloodworks Northwest, Seattle, WA, United States
Introduction: Donor-derived cell-free DNA (dd-cfDNA) emerged as a candidate biomarker for detecting graft injury, particularly from antibody-mediated rejection, and is currently proposed to complement donor-specific antibodies (DSAs) as an alloimmune-mediated injury surveillance method. We sought to investigate the impact of an initial increase of dd-cfDNA level on subsequent decline in graft function.
Materials: The study included all kidney transplant (KT) recipients that underwent dd-cfDNA testing as part of their clinical care between September 2017 and December 2019 at our center. Only patients with a follow-up of at least 12 months were included in this analysis. An elevated dd-cfDNA was defined as a level over 0.5%.
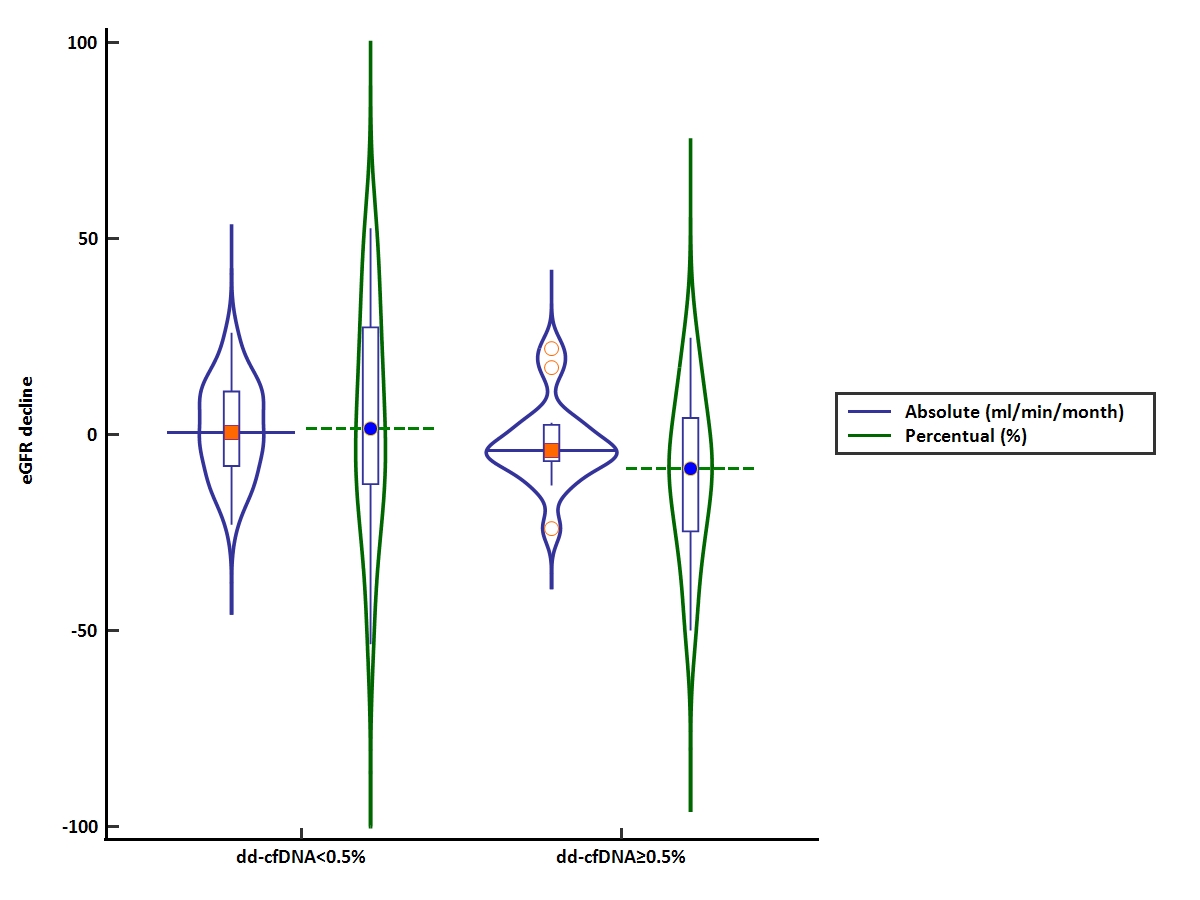
Results: Of the 171 KT recipients tested for dd-cfDNA, 49 were followed for at least 12 months since initial testing. The study cohort had an initial serum creatinine and eGFR of 1.64 ± 0.58 mg/dl and 48 ± 21 ml/min/1.73m2, respectively. Overall, the absolute and percentual decline of eGFR were -0.12 ml/min/month (IQR, -0.43 to 0.56) and -4.26% (IQR, -20.5% to 17%), with 26.5% and 10.2% of patients having an eGFR decline of more than 15% and 30%, respectively. Fifteen patients (30.6%) had an elevated dd-cfDNA (over 0.5%) and had a similar baseline allograft function compared to those without an elevated dd-cfDNA level. After a median follow-up period of 15.3 months (IQR:13.6-19), patients with elevated dd-cfDNA had a faster decline of eGFR [absolute eGFR decline, -0.21 ml/min/month (IQR, -0.36 to 0.22); percentual GFR decline, -8.7% (IQR, -25% to 4.4%)], compared to those without elevated dd-cfDNA [absolute eGFR decline, +0.03 ml/min/month (IQR, -0.5 to 0.6); percentual GFR decline, +1.14% (IQR, -14% to 28%)](p=0.09)(Figure 1). Similarly, there was a tendency for a higher percentage of patients with elevated dd-cfDNA for a decline of eGFR greater than 15% (33.3% vs. 23.5%, p=0.5) or 30% (13.3% vs. 8.8%, p=0.6), respectively. In addition, patients with DSAs and an elevated dd-cfDNA showed a greater percentual eGFR decline (-9.1±16.5%), compared to those with DSAs and normal dd-cfDNA (+3.3±21.1%), or to those without DSAs and normal dd-cfDNA (+2.3±18.05%). In multivariable logistic regression analysis, an elevated dd-cfDNA level was independently associated with the subsequent percentual decline of eGFR (OR, 0.96; 95%CI, 0.93 to 1.00; p=0.05).
Conclusion: We have shown that despite initial stable allograft function, a higher dd-cfDNA level associates with subsequent decline of eGFR. Thus, the initial identification of subclinical graft injury could associate with long-term graft outcomes, expanding the clinical utility of dd-cfDNA.

right-click to download
