Ad.26.COV2.S versus BNT162b2 or mRNA-1273 as a third dose in solid organ transplant recipients seronegative after 2-dose mRNA vaccination
Teresa Po-Yu Chiang1, Jennifer L Alejo1, Jonathan Mitchell1, Jake D Kim1, Aura T Abedon1, Andrew H Karaba2, Letitia Thomas1, Macey L Levan1,3, Jacqueline M Garonzik-Wang4, Robin K Avery2, Andrew Pekosz5, William A Clarke6, Daniel S Warren1, Aaron AR Tobian6, Allan B Massie1,7, Dorry L Segev1,7, William A Werbel2.
1Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States; 2Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States; 3Department of Acute and Chronic Care, Johns Hopkins University School of Nursing, Baltimore, MD, United States; 4Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States; 5Department of Molecular Microbiology and Immunology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States; 6Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States; 7Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States
Introduction: Heterologous vaccination ("mixing platforms") for the third (D3) dose of SARS-CoV-2 vaccine is a potential strategy to improve antibody responses in solid organ transplant recipients (SOTRs), but data are mixed as to whether this provides any differential immunogenicity benefit.
Method: We assessed for differences in immunogenicity and tolerability of homologous (BNT162b2 or mRNA-1273; D3-mRNA) versus heterologous (Ad.26.COV2.S; D3-JJ) D3 among 377 SARS-CoV-2-infection naïve SOTRs who were seronegative after two mRNA vaccines. We measured anti-spike titers and used weighted (age and organ type) Poisson regression to evaluate prevalence of seroconversion and development of high-titers, comparing D3-JJ to D3-mRNA, at 1, 3 and 6-months post-D3. Titers measured after a fourth dose of vaccine, receipt of monoclonal antibodies, or SARS-CoV-2 infection were excluded from analyses.
Results: 40 D3-JJ recipients and 337 D3-mRNA recipients were similar in sex, years since transplant, and mycophenolate use. At 1-month post-D3, 63% of D3-JJ recipients and 52% of D3-mRNA recipients became seropositive (p=0.28); this difference did not reach statistical significance in weighted analysis (weighted incidence-rate-ratio wIRR=0.981.291.69, p=0.064). High-titer response at 1-month post-D3 occurred in 29% of D3-JJ recipients and 25% of D3-mRNA recipients (p=0.7); this difference did not reach statistical significance in weighted analysis (wIRR=0.911.572.70, p=0.10). At 3-months post-D3, 80% of D3-JJ recipients and 57% of D3-mRNA recipients were seropositive (p=0.018); D3-JJ recipients were 1.4-fold more likely to be seropositive (wIRR=1.101.401.77, p0.006). High-titer response at 3-months post-D3 occurred in 27% of D3-JJ recipients and 22% of D3-mRNA recipients (p=0.64); this did not reach statistical significance (wIRR=0.440.921.93, p=0.8). At 6-months post-D3, 88% of D3-JJ recipients and 59% of D3-mRNA recipients were seropositive (p=0.026); D3-JJ recipients were 1.41-fold more likely to be seropositive (wIRR=1.041.411.93, p=0.029). High-titer response at 6-months post-D3 occurred in 59% of D3-JJ recipients and 21% of D3-mRNA recipients (p=0.005); D3-JJ recipients were 2.63-fold more likely to develop high-titers at 6-months post-D3 compared to D3-mRNA recipients (wIRR=1.382.635.00, p=0.003). Severe adverse reactions after D3 were rare; one D3-mRNA recipient reported fluid overload temporarily associated mRNA-1273. No D3-JJ recipients reported rejection before or after D3. Six D3-mRNA recipients reported acute rejection at median (IQR) 23 (12-131) days before D3.
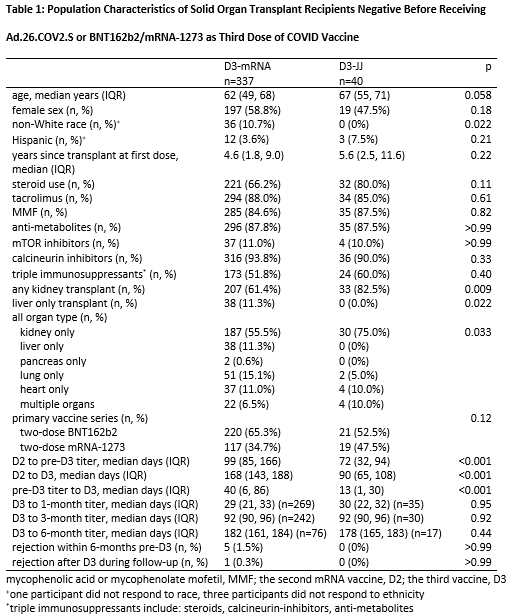
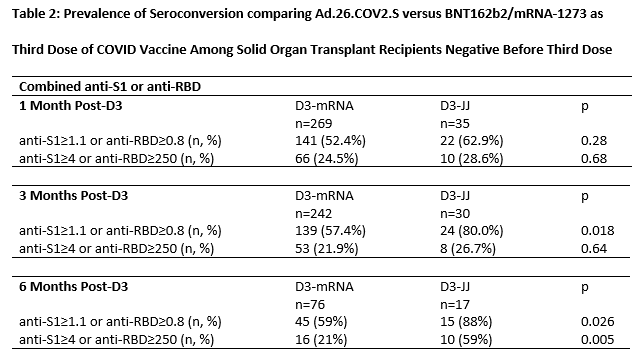
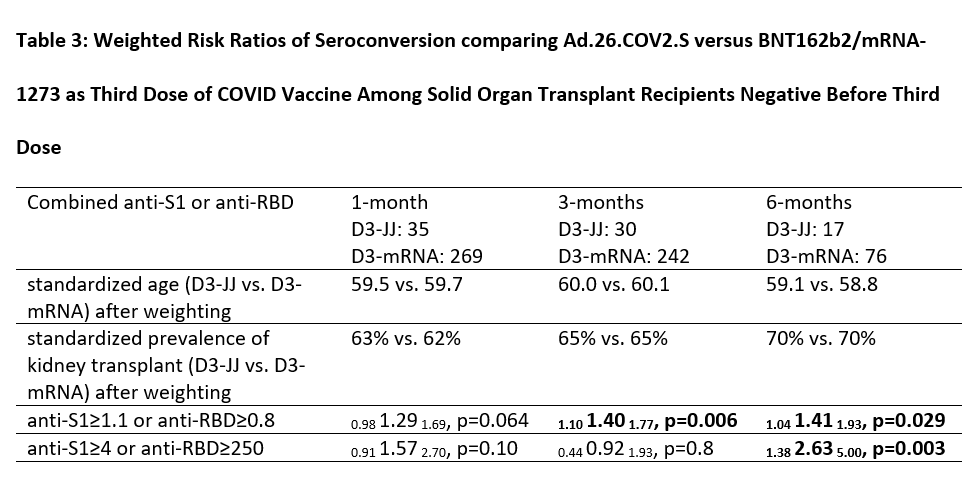
Conclusion: Heterologous vaccination with Ad.26.COV2.S for D3 was associated with higher late seroconversion than homologous vaccination with a third mRNA dose among SOTRs negative after a 2-dose mRNA series. SOTRs with persistent negative sero-response to mRNA vaccine series might benefit from Ad.26.COV2.S as an additional vaccine dose.
Ben-Dov family. Trokhan Patternson family. 5T32DK007713 (Dr. Alejo). ASTS Fryer Resident Scientist Award (Dr. Mitchell). K01DK101677 (Dr. Massie). K23DK115908 (Dr. Garonzik-Wang). K24AI144954 (Dr. Segev). U01AI138897 (Dr. Werbel). K23AI157893 (Dr. Werbel). DL Segev has the following financial disclosures: consulting and speaking honoraria from Sanofi, Novartis, CLS Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific, Regeneron, and AstraZeneca. Dr. Levan is the Social Media Editor for Transplantation. Dr. Avery has grant/research support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, Regeneron, and Takeda/Shire. The remaining authors of this manuscript have no financial disclosures or conflicts of interest to disclose.

right-click to download
