Hypothermic machine perfusion ameliorates fibrinolysis and microthrombi clearance after DCD kidney donation
Tamar AJ van den Berg1,2, Marius C van den Heuvel3, Stephan JL Bakker4, Cyril Moers1,2, Ton Lisman1,2, Robert A Pol1.
1Department of Surgery, University Medical Center Groningen, Groningen, Netherlands; 2Surgical Research Laboratory, Department of Surgery, University Medical Center Groningen, Groningen, Netherlands; 3Department of Pathology, University Medical Center Groningen, Groningen, Netherlands; 4Division of Nephrology - Department of Internal Medicine, University Medical Center Groningen, Groningen, Netherlands
Oxygenated hypothermic machine perfusion (HMP) is superior to static cold storage (SCS) in kidney transplantation. However, the mechanisms determining this superiority have only been studied to a limited extent. The aim of this study was to investigate whether HMP is superior because it provides a prolonged and better flush, removing microthrombi and fibrin depositions that may have accumulated in the kidney after circulatory arrest.
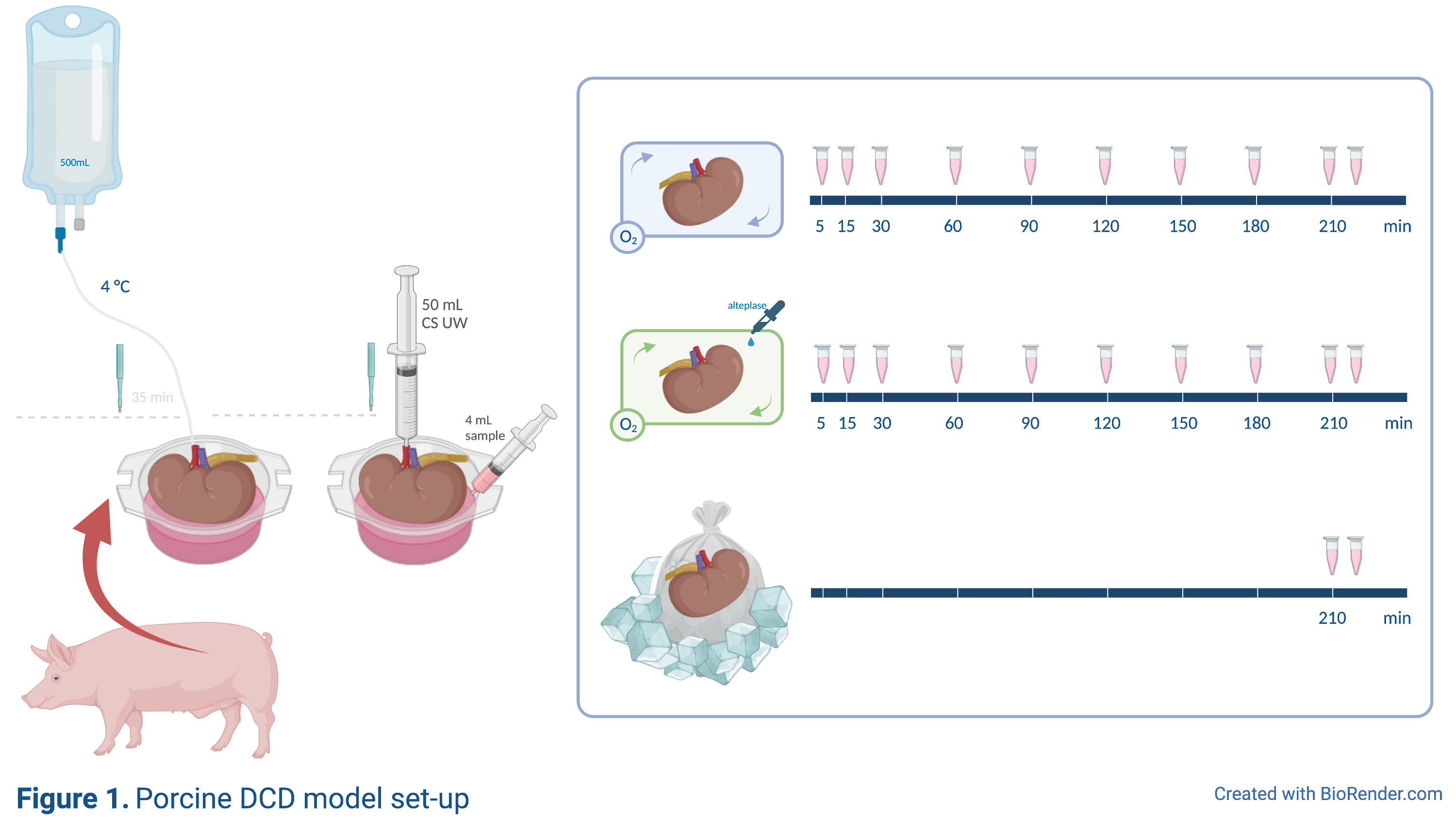
Viable porcine kidneys from an abattoir underwent 35 minutes of warm ischemia, were flushed with 500mL heparinized cold University of Wisconsin solution and subsequently preserved for 3.5 hours using either SCS, HMP, or HMP+alteplase (HMP+) (Figure 1). D-dimer, von Willebrand factor (VWF) and tissue-type plasminogen activator (tPA) levels were measured in perfusate samples at set times. The first and last perfusate sample prior to and after SCS/HMP was taken from a separate 50mL flush, directly from the renal vein. Kidney punch biopsies were immunohistochemically stained for an antibody against fibrin(ogen).
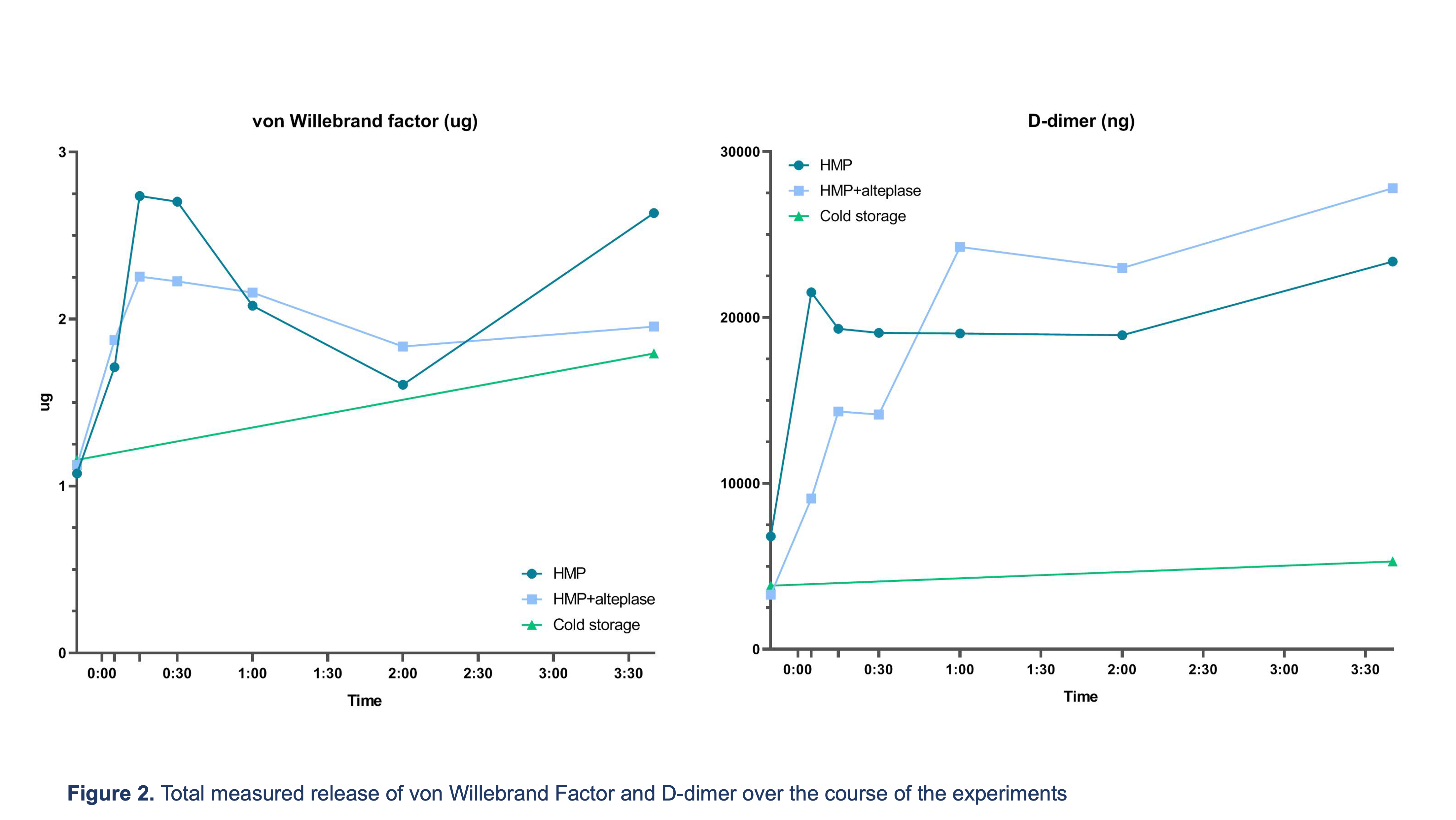
Warm and cold ischemic times were comparable between groups. The median [IQR] duration of the flush was 11 min [6–15] in SCS, 9 min [7–12] in HMP and 9.5min [7–11] in HMP+. There was no significant difference between total VWF release into the perfusate between groups after storage (SCS 1.65 ug [1.28–3.30] vs. HMP 1.87 [1.11–4.00] vs. HMP+ 1.70 [1.11–3.06] p=0.80, Figure 2).
However, total D-dimer release was much higher in the HMP and HMP+ groups compared to SCS (24,650 ng [2,380–38,164] and 25,840 [9,010–45,050] ng vs. 6,325 [2,100–7,550], p=0.09 and p<0.01, respectively). In SCS treated kidneys there was a small increase in microthrombi over time (0.30 microthrombi/mm2 [0.22–1.39] to 0.73 [0.22–1.08]), while there was a decrease in the number of microthrombi in HMP- (0.91 [0.07–2.30] to 0.57 [0.19–1.34]) and HMP+ (0.63 [0.43–1.24] to 0.34 [0–0.87]) preserved kidneys.
Our results suggest that the superiority of oxygenated HMP is at least partly caused by a better flush to clear the kidney graft from donor-derived thrombi, considering an increased release of D-dimer, and a reduction of microthrombi over time. This phenomenon has already been observed in machine-perfused human livers. Furthermore, HMP does not significantly increase endothelial activation, as evidenced by low levels of VWF in the perfusate. Addition of a thrombolytic agent to an HMP-fluid does not seem to be of added value.
Tekke Huizinga Foundation (grant number SH-197).

right-click to download
