qPCR based immunotherapy in intestinal transplant recipients and candidates
Maria Lasa1,2, Esther Ramos-Boluda3, Javier Serradilla3, Ane Miren Andrés4,5, Pilar Serrano3, Francisco Hernández Oliveros4,5, Estela Paz-Artal1,2, Paloma Talayero1,2.
1Department of Immunology, University Hospital 12 de Octubre, Madrid, Spain; 2Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), University Hospital 12 de Octubre, Madrid, Spain; 3Unit of Intestinal Rehabilitation and Transplant, University Hospital La Paz, Madrid, Spain; 4Department of Pediatric Surgery, University Hospital La Paz, Madrid, Spain; 5IdiPaz Research Institute, University Hospital La Paz, Madrid, Spain
Introduction: Inflammatory bowel disease (IBD) in intestinal transplantation (ITx) recipients and candidates is a complication that may be the result of an immune dysregulation due to the immunosuppression or the underlying disease. Conventional treatment of IBD, including corticosteroids and immunotherapy with monoclonal antibodies, uses to be effective, but in some patients the inflammation is refractory to the treatment. The aim of the study was to determine the intestinal mucosa cytokine expression in order to establish the optimal therapy in two multivisceral recipients and two ITx candidates with persistent enteritis.
Methods: Two multivisceral recipients with episodes of ulcerative enteritis refractory to corticosteroids and infliximab treatment were studied, as well as, two ITx candidates with intestinal failure and inflammatory colitis and enteropathy, respectively. Mucosal biopsies were taken from affected and healthy areas and frozen in RNAlater until RNA extraction. Expression of cytokine genes which are targeted by approved drugs (IL1B, IL6, IL12p70, IL17A, IL23 and TNF) was determined by qPCR. Gene expression was calculated as 2-ΔCt using GAPDH as endogenous control.
Results: ITx recipients (Patients 1 and 2): both patients (13 and 14 years old males) developed in the late posttransplant ulcerative ileitis and ulcerative colitis respectively. They were initially treated with metilprednisolone without achieving endoscopic improvement. Graft cytokine expression in pre-treatment biopsies of healthy and inflamed area was carried out, both of them showing a significant increase of IL6, correlating with high IL6 plasmatic levels (Figure 1A and 1B). Tocilizumab was then administered every two weeks, getting the clinical resolution in two months.
ITx candidates: Patient 3 was a 5 years old male with trichohepatoenteric syndrome, showing ulcerative colitis. He was tread with corticosteroids without endoscopic or clinical improvement. Intestine cytokine expression in pre-treatment biopsies was carried out objectifying elevated IL1 and IL6 levels, so he was treated with Tocilizumab achieving great healing of the colitis (Figure 1C). Patient 4 was a 1 year old male suffering from autoinflammatory disorder with a mutation in mevalonate kinase gene, having enteropathy. He was empirically treated with Infliximab with initial improvement, but having a deterioration afterwards. Intestine cytokine expression in pre-treatment biopsies showed elevated IL1, IL6 and TNF levels (Figure 1D). He was treated with Anakinra without clinical improvement, changing for Adalimumab achieving clinical resolution.
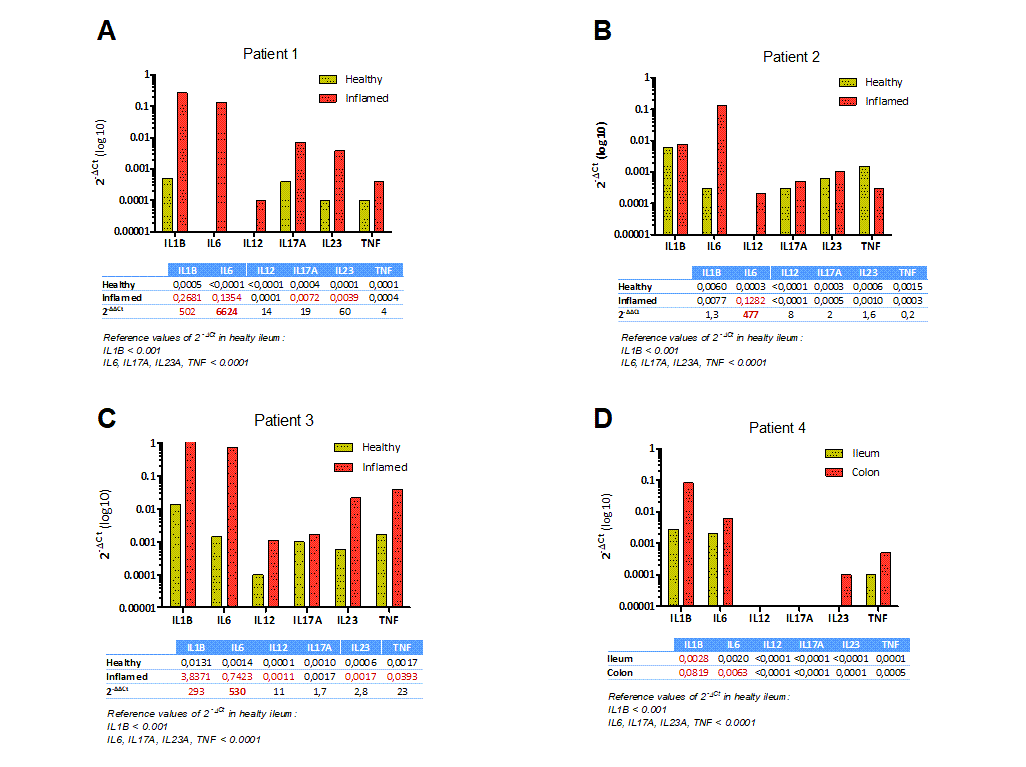
Conclusions: In the context of mucosal inflammation, the analysis of intragraft cytokine expression by qPCR is a useful and affordable tool to guide the treatment in a personalized way, showing a high effectiveness of the therapy.

right-click to download
