Dr. Catalina Alvarez completed a Pediatric Nephrology fellowship at Hospital Infantil de Mexico, Federico Gómez, while earning a master’s degree in Medical Sciences from the National Autonomous University of Mexico, she is now a Ph.D. Medical Sciences candidate at the same university. Dr. Alvarez received further training in pediatric transplantation at the Hospital for Sick Children Transplant & Regenerative Medicine Centre in Toronto and is currently a Ph.D. student in Clinical Epidemiology at the University of Toronto Institute of Health Policy Management and Evaluation.
Dr. Alvarez’s research interest is in pharmacogenetics, pharmacoepidemiology and individualization of therapy. Her Ph.D. dissertation is focused on the relationship between long-term drug-related toxicity and the development of comorbidities in pediatric solid organ transplant recipients. Dr. Alvarez has researched the influence of polymorphisms related to dosing and effectiveness in long-term immunosuppression. She has published on drug disposition, drug monitoring and pharmacokinetics in children.
Dr. Alvarez has a particular interest in measuring healthcare transition from pediatric to adult-focused services and has assisted in translating readiness tools into Spanish, generating data to guide clinical practice. Dr. Alvarez has co-authored several publications in this field and aims to improve clinical research in developing countries.
Influence of tacrolimus CYP3A5 polymorphisms on the dose-level response after pediatric kidney transplantation
Ana Catalina Alvarez-Elias1, Pilar Garcia-Roca1, Luis Velasquez-Jones2, Saul Valverde-Rosas2, Gustavo Varela-Fascinetto3, Mara Medeiros-Domingo1.
1Unidad de Investigación y Diagnóstico en Nefrologia y Metabolismo Mineral Oseo, Hospital Infantil de Mexico, Federico Gomez, Delegacion Cuahutemoc, , Mexico; 2Departamento de Nefrologia, Hospital Infantil de Mexico, Federico Gomez, Delegacion Cuahutemoc, , Mexico; 3Cirugia de Trasplante, Hospital Infantil de Mexico, Federico Gomez, Delegacion Cuahutemoc, , Mexico
Background: Tacrolimus exhibits wide inter and intra-patient variability. CYP3A5 tacrolimus polymorphisms predict its metabolization. We aim to identify the influence of polymorphisms on the pharmacological parameters. The present report is part of the exploratory data analysis of a pediatric trial that used the initial tacrolimus dose according to the CYP3A5 genotype in kidney transplant recipients.
Methods: We performed an exploratory data analysis of pharmacological parameters from a single-center, non-randomized, open-label clinical trial in children from 0-to 18 years of age who received a kidney transplant at the Hospital Infantil de México, Federico Gómez from January 2013 to December 2018. All children were genotyped before transplantation and assigned to one of two groups: the Genotype-guided group, which initial dose was according to the CYP3A5 polymorphisms and the Conventional-dose group (see abstract AID-1780), with further dose adjustments on the decision of the clinicians. We followed them for six weeks and recorded tacrolimus dose received, trough level achieved, deltas of dose and trough levels between time points (weekly).
Results: We included 81 subjects; 40 received the genotype-guided dose, the mean age at transplantation was 13.3 y/o, 51.8% were female. After six weeks of transplantation, linear regressions showed no linear associations between tacrolimus dose and levels in the genotype-guided dose group (r2=0.0003, p=0.78) or the conventional-dose group (r2=0.0008, p=67). However, there were linear associations between the delta-dose and delta-levels in the genotype-guided dose group (r2=0.138, p=<0.0001) and conventional-dose group (r2=0.081, p=<0.0001). The subgroup analysis by genotype showed that AG1*3 polymorphism had a linear association between the tacrolimus delta-dose and delta-level (r2=0.168, p=<0.001). Furthermore, It reached statistical significance in the tacrolimus dose vs level analysis (r2=0.0308, p= 0.02). The GG3*3 polymorphism showed an association only in the delta-dose vs delta-level analysis (r2=0.057, p=<0.001), while the AA1*1 genotype did not show any linear associations between tacrolimus dose vs trough level or deltas (r2=0.006, p=0.64 and r2=0.108, p=0.08 respectively) (Figure).
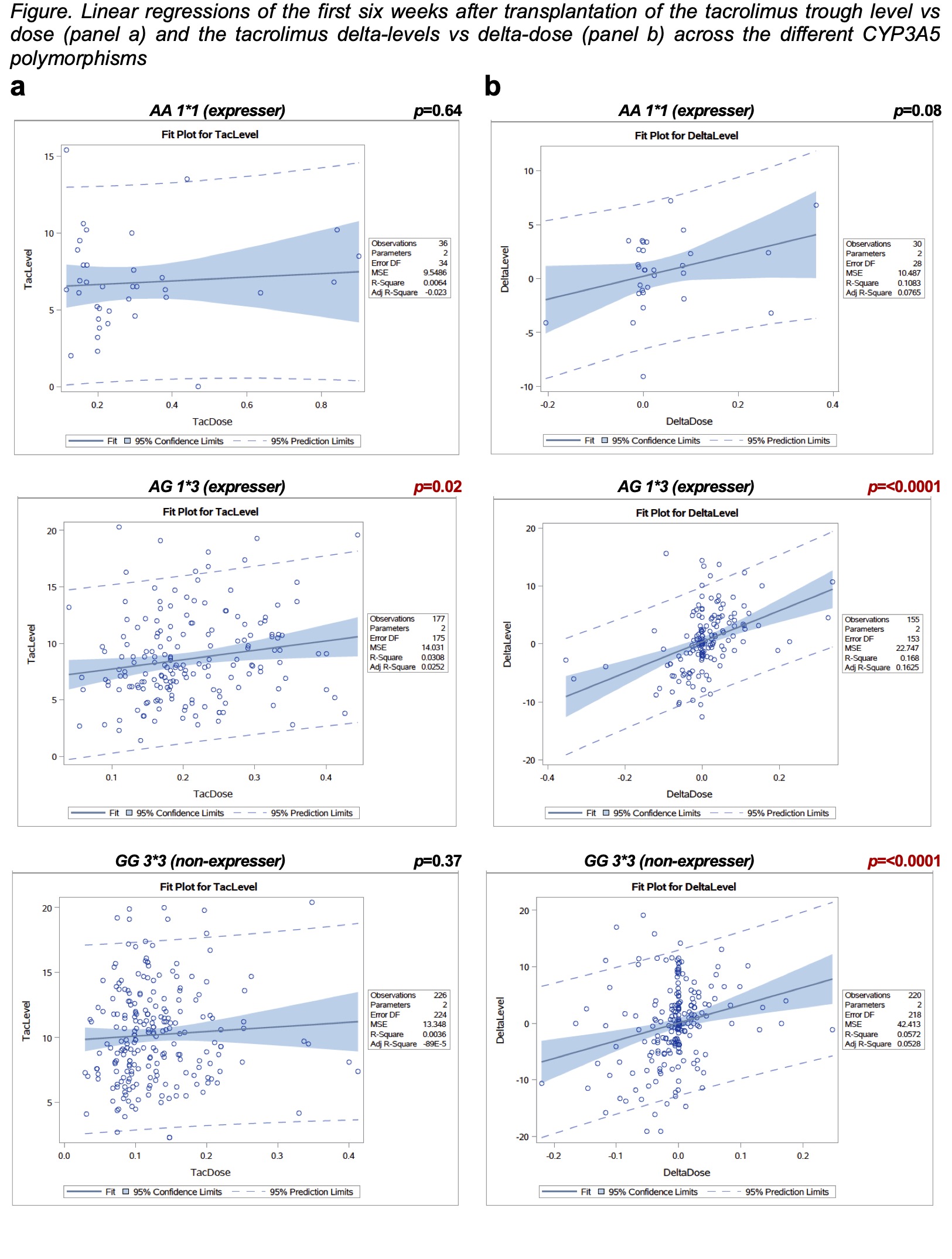
Conclusion: The tacrolimus delta-dose and delta-level across the different polymorphisms translate the clinician's decisions. The trough levels' response to dose adjustments might be guided by the CYP3A5 genotypes, specifically the AG1*3. Further studies are needed to estimate more accurate associations.

right-click to download
