Dr. Catalina Alvarez completed a Pediatric Nephrology fellowship at Hospital Infantil de Mexico, Federico Gómez, while earning a master’s degree in Medical Sciences from the National Autonomous University of Mexico, she is now a Ph.D. Medical Sciences candidate at the same university. Dr. Alvarez received further training in pediatric transplantation at the Hospital for Sick Children Transplant & Regenerative Medicine Centre in Toronto and is currently a Ph.D. student in Clinical Epidemiology at the University of Toronto Institute of Health Policy Management and Evaluation.
Dr. Alvarez’s research interest is in pharmacogenetics, pharmacoepidemiology and individualization of therapy. Her Ph.D. dissertation is focused on the relationship between long-term drug-related toxicity and the development of comorbidities in pediatric solid organ transplant recipients. Dr. Alvarez has researched the influence of polymorphisms related to dosing and effectiveness in long-term immunosuppression. She has published on drug disposition, drug monitoring and pharmacokinetics in children.
Dr. Alvarez has a particular interest in measuring healthcare transition from pediatric to adult-focused services and has assisted in translating readiness tools into Spanish, generating data to guide clinical practice. Dr. Alvarez has co-authored several publications in this field and aims to improve clinical research in developing countries.
Effect of CYP3A5 genotype-guided versus conventional initial dose of tacrolimus in children with kidney transplants
Ana Catalina Alvarez Elias1, Maria del Pilar Garcia-Roca1, Luis Velasquez-Jones2, Saul Valverde-Rosas2, Gustavo Varela-Fascinetto3, Mara Medeiros-Domingo1.
1Unidad de Investigación y Diagnóstico en Nefrologia y Metabolismo Mineral Oseo, Hospital Infantil de Mexico, Federico Gomez, Delegacion Cuahutemoc, , Mexico; 2Departamento de Nefrologia, Hospital Infantil de Mexico Federico Gomez, Delegacion Cuahutemoc, , Mexico; 3Cirugia de Trasplante, Hospital Infantil de Mexico Federico Gomez, Delegacion Cuahutemoc, , Mexico
Background: CYP3A5 tacrolimus polymorphisms predict its metabolization. We aimed to test the advantage of a genotype-guided starting dose after kidney transplantation to reduce the time to target drug levels and improve allograft surveillance.
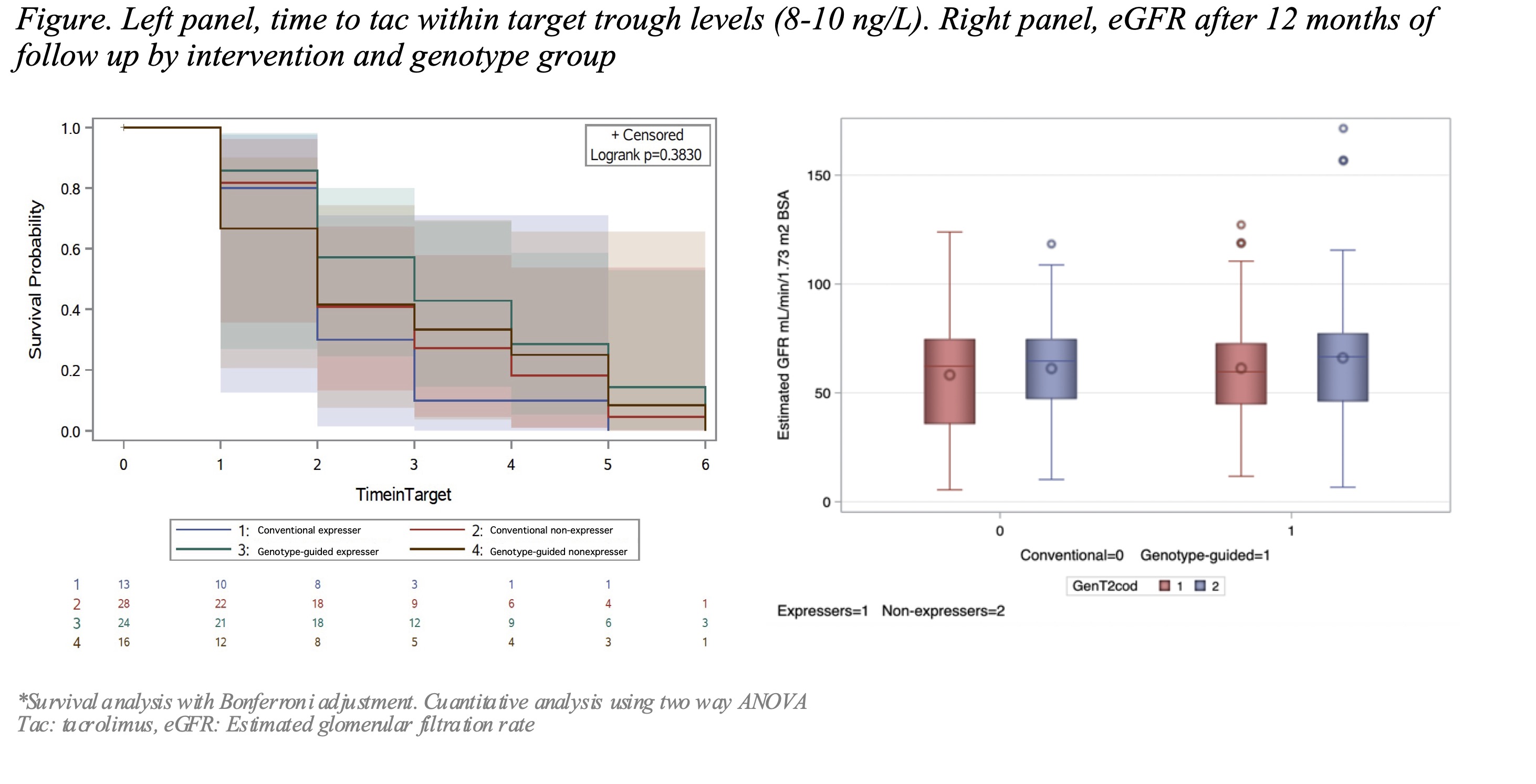
Methods: We performed a single-center, open-label parallel group, randomised controlled trial in kidney recipients (0-18 y/o) at the Hospital Infantil de México, Federico Gómez, from Jan-2013 to Dec-2018. Patients were assigned to one of two groups. The genotype-guided group received the dose according to the CYP3A5 polymorphisms (AA1*1, AG1*3 [expressers], and GG3*3, [non-expressers]). The Conventional-dose group received standard of care. We followed them for 12 months registering time to target levels, estimated glomerular filtration rate (eGFR), rejection, and nephrotoxic events.
Results: We included 81 patients; 40 received the genotype-guided dose, the mean age at transplantation was 13.3 y/o, 51.8% were female. There was a higher frequency of expressers in the genotype-guided than the conventional-dose group (29.6 Vs 16.1% p=0.04), having a higher number of AA1*1. There were no differences in the time to achieve levels within-target (8-10 ng/L) logrank=0.182 between groups. There were no associations with rejection, OR 0.86 (95% CI 0.26-2.84, SE 0.606), or nephrotoxicity events, OR 5.85 (95% CI 0.60-56.8, SE 1.161) and the intervention. The eGFR remained similar (63.1 27.4 Vs 60.3 22.3 mL/min/1.73 m2 SC p=0.32) among groups. The subgroup analysis by genotype showed that expressers in the genotype-guided dose group took longer to achieve target levels (mean 5 vs 3.2 weeks, log-rank= 0.383).
Conclusions: Tacrolimus CYP3A5 genotype-guided initial dose after kidney transplantation did not show benefits on time to target levels, rejection, tac related nephrotoxic events or eGFR after a 12-months follow-up in our cohort. The higher frequency of expressers in the genotype-guided group could influence the results, showing a high impact of the polymorphisms. New dosing strategies are needed to improve accuracy.

right-click to download
