
Very low dose Anti-Thymocyte Globulins versus Basiliximab in non-immunized kidney transplant recipients
Christophe Masset1,2, Clarisse Kerleau1, Gilles Blancho1,2, Maryvonne Hourmant1,2, Diego Cantarovich1, Aurélie Houzet1, Magali Giral1,2, Claire Garandeau1, Jacques Dantal1,2.
1Service de Néphrologie et Immunologie Clinique, Institut de Transplantation Urologie Néphrologie , Nantes, France; 2Center for Research in Transplantation and Translational Immunology, UMR 1064, Université de Nantes, Nantes, France
DIVAT Nantes Consortium.
Background: The choice between Basiliximab (BSX) or Anti-Thymocyte Globulins (ATG) as induction therapy in non-immunized kidney transplant recipients remains uncertain. Whilst ATG can permit steroid withdrawal and CNI decrease, it also increases the risk of viral reactivations due to a prolonged dose-dependent lymphopenia. We compared transplant outcomes in non-immunized patients receiving a very low dosage of ATG (75 mg daily twice) versus BSX as induction therapy.
Methods: We included non-immunized patients receiving a first kidney transplant between 2015 and 2020. Studies outcomes were patient and graft survival, cumulative probabilities of acute rejection, infectious episode, CMV infection and Post-Transplant Diabetes (PTD). Cox, logistic or linear statistical models were used depending on the studied outcome and models were weighted on propensity scores.
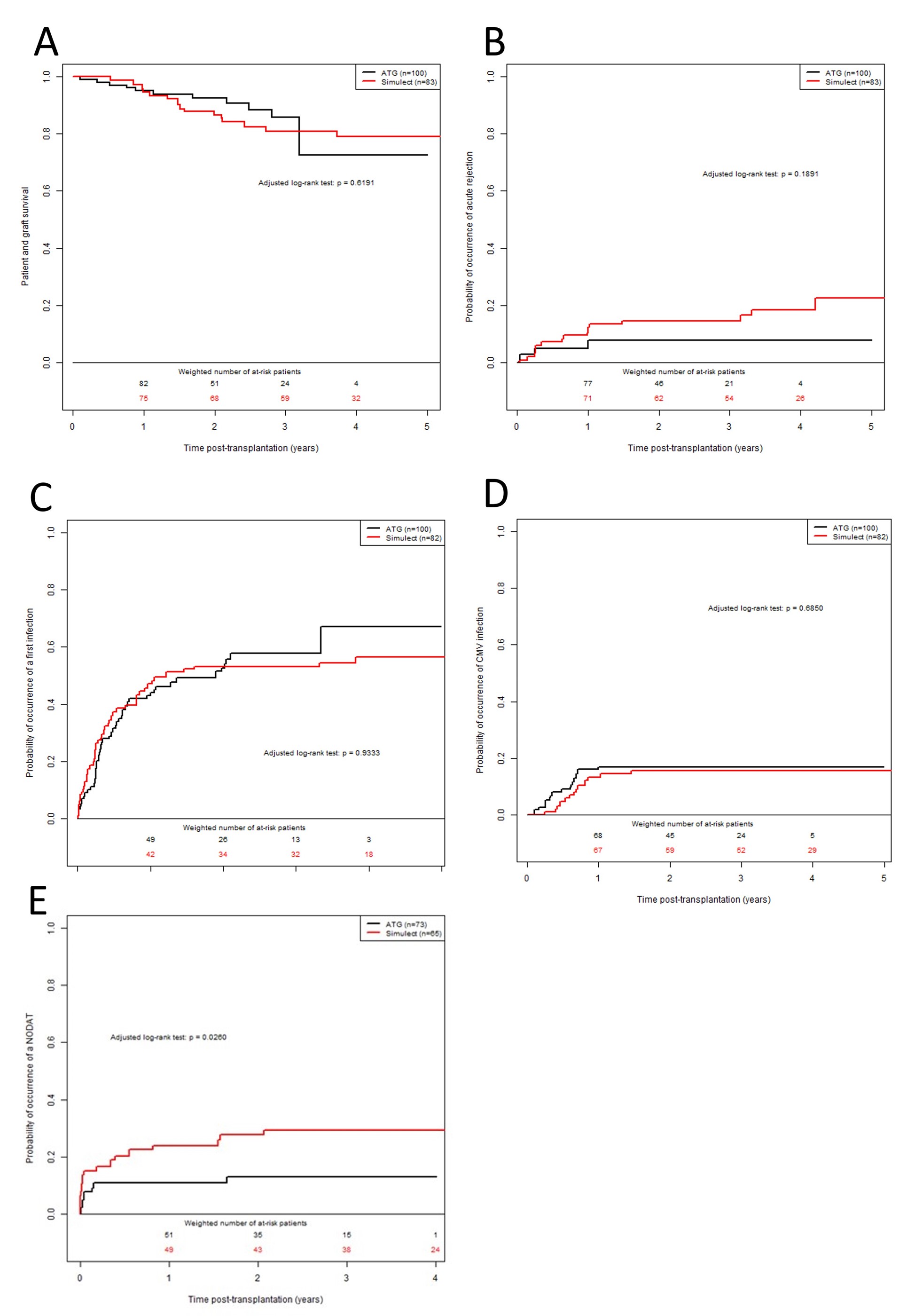
Results: 183 patients were included, 100 receiving ATG and 83 receiving BSX. Maintenance therapy was comparable between groups. Patient and graft survival did not differ between groups, as infectious and CMV episodes. There was a trend to a lower occurrence of BPAR in the ATG group (HR at 1.92; 95% CI: 0.77; 4.78, p-value = 0.1598). Occurrence of PTD was significatively higher in the BSX group (HR at 2.44; 95% CI: 1.09; 5.46; p-value = 0.0304), due to a high number of treated rejection episodes (14.5% vs 7%).
Conclusion: Induction with a very low dose of ATG in non-immunized recipients is safe, decreasing the occurrence of treated rejections episodes compared to BSX, thus leading to a lower rate of PTD.

right-click to download
