
Unraveling the stage-shift of acute rejection in renal allografts
Reuben Sarwal1, Wanzin Yazar1, Nick Titzler1, Jeremy Wong1, Chih-Hung Lai1, Christopher Chin1, Minnie Sarwal1, Srinka Ghosh1.
1NephroSant, San Mateo, CA, United States
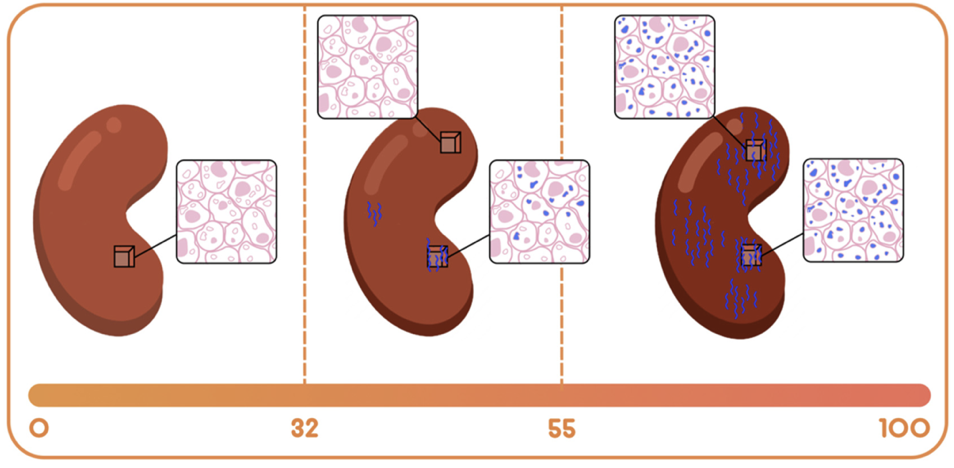
Introduction: The lack of accuracy in current allograft monitoring methodologies for allograft monitoring underscores a critical unmet need for more accurate immunosurveillance to uncover the flux in alloimmunity. QSant – a non-invasive, urine based multi-analyte diagnostic test – was clinically validated to prognosticate injury, risk of evolution, and resolution of acute rejection. QScore—the composite score across measurements of DNA markers: the amount of cell-free DNA, fraction of methylated cfDNA, protein and metabolic biomarkers in the QSant assay—enables this risk prognostication. This study now explores the clinical utility of QSant across the alloimmunity gradient of 32–100 for the early diagnosis of allograft injury and rejection.
Methods: Utilizing a large real-world dataset of contemporaneous kidney allograft biopsy and QSant data from 11 US kidney transplant centers, the QScore offers a dynamic view into the changing state of allograft injury and rejection in response to alterations in the patients’ immunosuppression regimen. First analysis included Q-Score distributions in the biopsy-paired validation (n = 162) and prediction (n = 91) data from the Yang cohort, which were collected between 2010 and 2018 [10]. Second was a new analysis of a real-world data (RWD) cohort, comprising of prospectively collected urine samples (n = 235).
Results: The RWD cohort (n = 235) of both adult and pediatric renal transplant patients demonstrated a spectrum across the Q-Score distribution. Specifically, 37.9% of the samples were enriched for immune quiescence (Q-Score < 32); the remaining 62.1% were enriched for the injury/rejection spectrum (Q-Score ≥ 32). The QScore <32 classifies stable allograft recipients; QScore between 32-55 exposes a state of alloimmunity flux; and the score between 55-100 provides advanced alloimmunity. Serial monitoring by QSant across a subset of 32 patients (RWD cohort,) over a period of two consecutive quarters (timepoints 1 and 2), captures the essence of the alloimmunity flux (p-value: 0.086 by non-parametric Wilcoxon test). Patients in the immunoquiescent domain (31% [10/32]) by and large continue to be stable (with Q-Scores that track at <32) 22% [7/32]). However, 9.4% (3/32) of patients show Q-Score drifts above 32 with evidence of early rejection. Patients in the alloimmune injury domain follow three trajectories: i) a planar gradient in QScore across time, showing persistence of alloimmunity; ii) an increasing gradient, delineating patients moving into the mature acute rejection domain; iii) a decreasing gradient, delineating patients moving into the immunoquiescent domain, putatively in response to immunomodulation following assessment of active rejection (Figure 1).
Conclusion: The observed alloimmunity flux suggested precision monitoring with QSant has the potential to differentiate higher-grade acute rejection episodes from sub-clinical rejection, thereby improving a transplant recipient’s’ quality of life.

right-click to download
