
QSant, a multi-analyte urine-based test, types the injury of DGF and its recovery
Helio Tedesco1, Reuben Sarwal3, Monica Nakamura1, Nicholas Titzler3, Yasmin Dreide1, Wanzin Yazar3, Giovana Bittencourt1, Jose O.M Pestana1, Srinka Ghosh3, Minnie Sarwal2.
1Transplante Renal-Disciplina de Nefrologia, Hospital do Rim-Escola Paulista de Medicina-UNIFESP, São Paulo, Brazil; 2Department of Surgery , UCSF, San Francisco, CA, United States; 3NephroSant, San Mateo, CA, United States
Introduction: QSant, a urine-based test quantitates alloimmune injury(AI) and acute rejection (AR) in the allograft via analysis of 6 biomarkers - cfDNA, methylated cfDNA, total protein, CXCL10, clusterin and creatinine. It utilizes a machine learning based algorithm for prognostication of injury and rejection risk. We propose the application of QSant to monitor AI in DGF and its recovery. Also use QSant as a surrogate to biopsy and refine the need for biopsy when the rejection risk is high.
Methods: 65 HLA-DR matched, renal allograft recipients, on TAC/MMF/prednisone, had 431 serial urine samples collected for QSant, over the 3 months post-transplant(MPT). All patients received a single 3mg/kg dose of anti-thymocyte globulin within 24 hours of graft re-vascularization. Clinical DGF was observed in 57% of the cohort. 62/65 (92%) of patients had a biopsy in the first month post-tx, with 80% of biopsies in the first week. Of the clinical DGF patients, 13% received a surveillance biopsy. Only 12% of biopsies had histological AR, others showed AI. QSant was used for quantification of AI (Q-Score >32) or immunequiescence (Q-Score <32) and a time-series analysis was performed. A PCA probed individual biomarker contributions in DGF patients to determine if specific biomarkers could predict recovery from IRI.
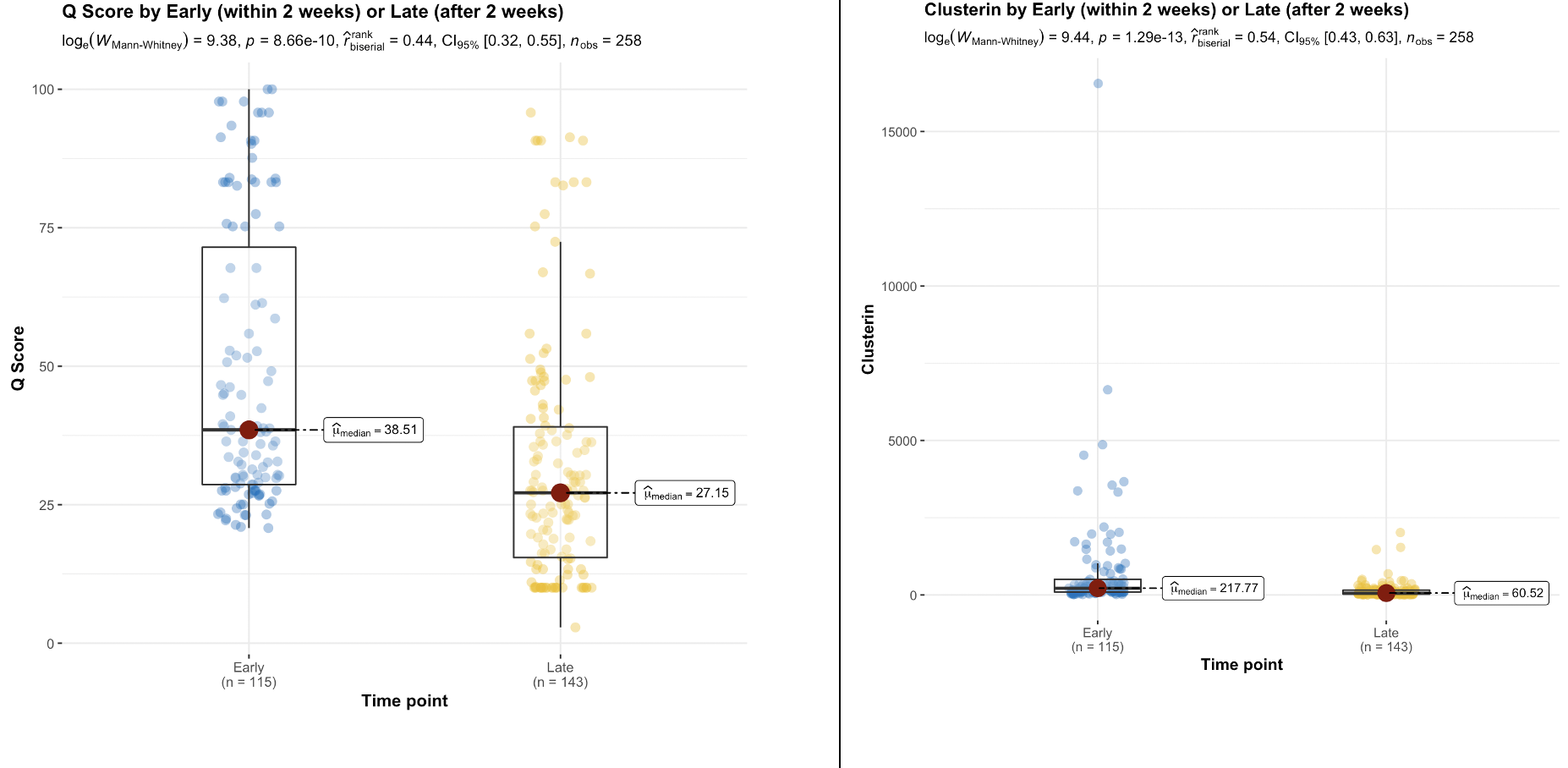
Results: The QSant biomarker profile for BPAR is significantly different from pure DGF without AR. A multivariate logistic regression of BPAR - with median Q-Score: 59(95% CI 50 – 69) - established cfDNA to be most significant (p=0.0029). QSant time-series analysis showed a significant(p=5.8e-19) decline from AI (Q-Score:47) to allograft stability (QScore:13) over the 3 MPT. The associated 30% decline in Q-Score indicative of allograft stability was also significant (p=8.66e-10). A significant decline (p=0.004) in rejection risk with Q-Score dropping into immunequiescence was observed after the first 2 weeks. In patients with recovery from DGF, concomitant with Q-Score there was a 3.6x (p=9.4e-6), 3x (p=1.42e-5) and 3x(p=0.000243) decline in clusterin, total protein and CXCL10, respectively. In the DGF sub-cohort that recovered function, PCA showed Clusterin explained 52% of the variance. For the samples with no histological evidence of rejection (n = 21), 90%(n=19) of the biopsies were done within the first two weeks post-transplantation. 36% of these had a Q-Score > 55: cases where QSant identified potentially evolving rejection missed by biopsy. 26% had Q-Scores in the immunequiescent range: samples where QSant might obviate the need for a biopsy.
Conclusions: Serial monitoring with QSant captures cases of DGF with concomitant AR and the early onset and resolution of DGF-related renal injury. QSant confirms recovery of allograft IRI in 90% of patients at 3 mo post-tx. Persistently elevated Q-Scores in a small subset of DGF patients suggests closer monitoring of these patients for increased risk of AR and chronic allograft injury over time.

right-click to download
