
Tacrolimus withdrawal after mesenchymal stromal cell therapy is associated with donor-specific antibody formation in kidney transplant recipients
Suzanne Bezstarosti1,2, Marlies E. J. Reinders2, Soufian Meziyerh2,3, Kim H. Bakker1, Dave L. Roelen1, Johan W. de Fijter2,3, Sebastiaan Heidt1,4.
1Immunology, Leiden University Medical Center, Leiden, Netherlands; 2Internal Medicine (Nephrology), Leiden University Medical Center, Leiden, Netherlands; 3LUMC Transplant Center, Leiden University Medical Center, Leiden, Netherlands; 4Eurotransplant Reference Laboratory, Leiden, Netherlands
Introduction: Recently, the clinical phase II Triton study demonstrated that mesenchymal stromal cell (MSC) therapy is a safe method to facilitate tacrolimus withdrawal in kidney transplant recipients (KTRs). Here, we analyzed de novo donor-specific antibody (dnDSA) formation and HLA molecular mismatch in MSC-treated KTRs.
Methods: All patients underwent a first, living-donor kidney transplantation and received alemtuzumab induction, steroids, tacrolimus and everolimus. Patients in the MSC arm of the study received two infusions of autologous MSC at week 6 and 7 post-transplantation. Subsequently, tacrolimus was tapered and completely withdrawn at week 8. Patient sera were tested by luminex single antigen bead (SAB) assays (Immucor) for dnDSA assignment; positivity was defined as MFI > 1000. DSA were further characterized using a modified SAB assay to detect IgG1, IgG2, IgG3 and IgG4 subclasses. Complement binding was assessed with a C3d assay (Immucor). Patients and donors were HLA typed on 11 loci by next-generation sequencing. Eplet mismatches were calculated using HLAMatchmaker 2.0.
Results: In total, 3 out of 27 patients (11%) in the control group and 11 out of 29 patients (38%) in the MSC group developed dnDSA, of which the majority was directed against HLA-DQ. The majority of DSA were complement binding and had different IgG subclasses. Although patients in the MSC group had an increased rate of dnDSA formation, the degree of antigen mismatch was similar to the control group (Figure 1). Cox regression showed that both MSC treatment group (HR=4.247, P=0.032) and HLA class II antibody-verified eplet mismatch (HR=1.176 per single eplet mismatch, P=0.001) were independently associated with dnDSA development. Despite the increased dnDSA formation in MSC-treated patients, incidence of rejection or graft loss was not increased and eGFR was stable at 2 years follow-up (Figure 2).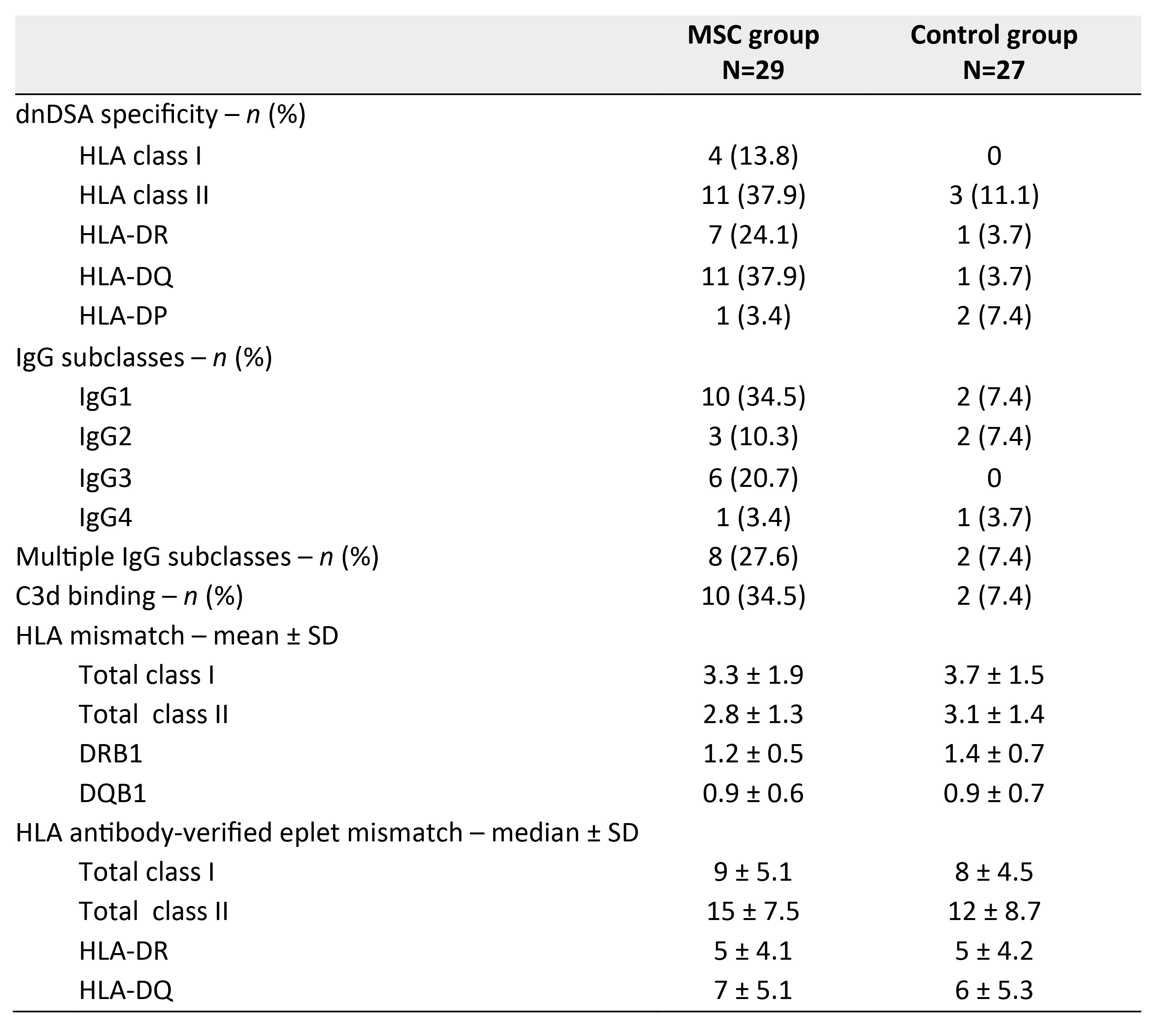
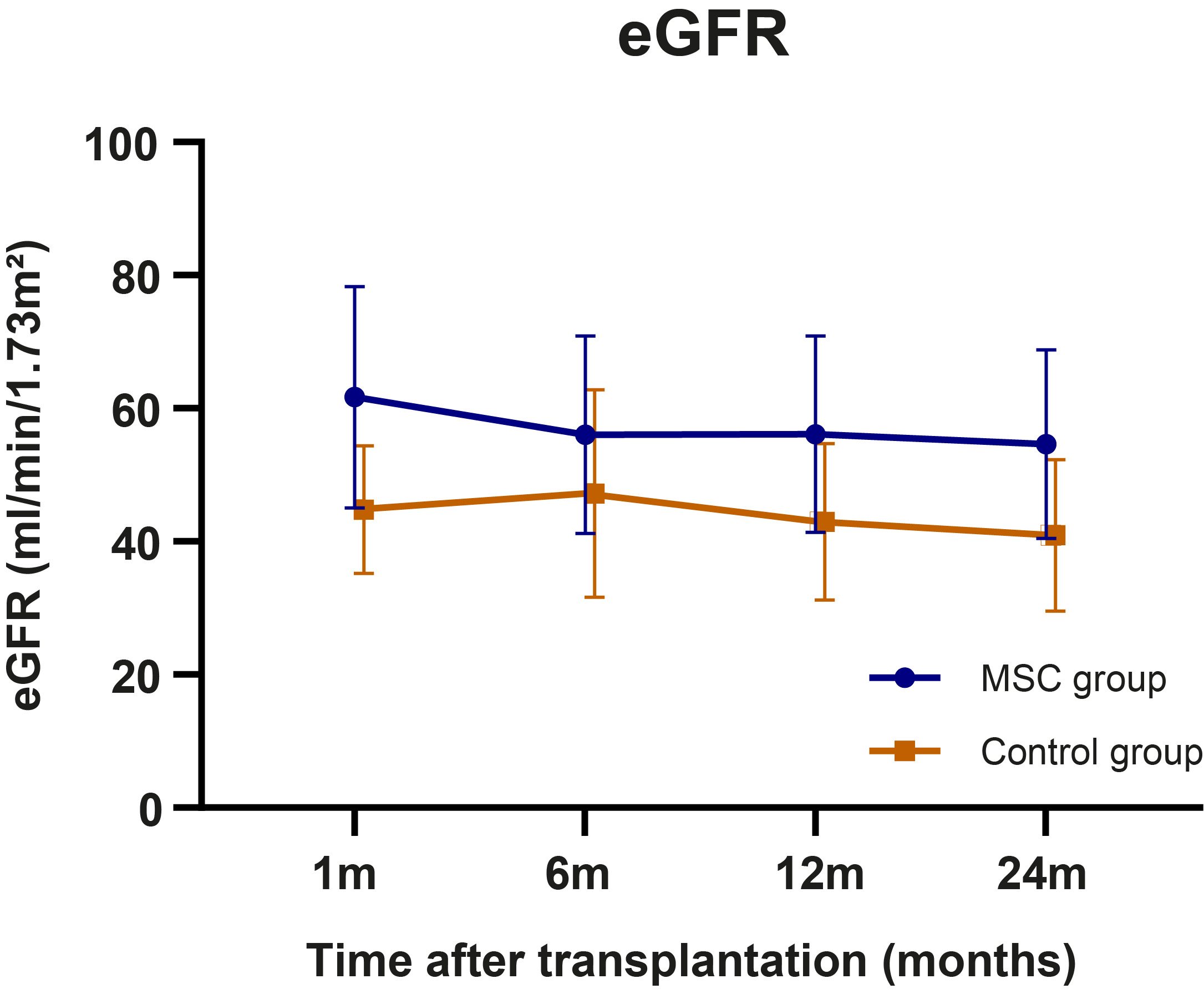
Conclusion: Tacrolimus withdrawal after MSC therapy is associated with an increased rate of dnDSA formation in KTRs. However, no increased risk of rejection or inferior graft function was found, raising the question on the pathogenicity of these DSA, despite the presence of complement fixing IgG subclasses. We found that patients with a high HLA class II eplet mismatch load were less likely to tolerate tacrolimus withdrawal without developing dnDSA, which is in line with previous findings that HLA eplet mismatch load modulates tacrolimus through levels required to prevent dnDSA formation. Further research is warranted to explore HLA molecular mismatch load as a biomarker to guide personalized immunosuppression in transplantation.
Jon J van Rood Transplantatiefonds.

right-click to download
