
Enhanced histological yield and actionable findings when biopsy is guided by donor-derived cell-free DNA (dd-cfDNA)
Matthew Weir2, Didier Mandelbrot4, Jonathan Bromberg3, Shikha Mehta5, Nikhil Agrawal1, Grigory Shekhtman1, Wenlan Tian1, Edmund Huang6, Emilio Poggio7, Matthew Cooper8.
1Kidney Transplant, CareDx, Brisbane, CA, United States; 2Medicine, University of Maryland, Baltimore, MD, United States; 3Surgery, University of Maryland, Baltimore, MD, United States; 4Medicine, University of Wisconsin, Madison, WI, United States; 5Medicine, University of Alabama, Birmingham, AL, United States; 6Medicine, Cedars Sinai Medical Center, Los Angeles, CA, United States; 7Medicine, Cleveland Clinic, Cleveland, OH, United States; 8Surgery, Georgetown University School of Medicine, Baltimore, MD, United States
Introduction: Implementation of molecular biomarkers in kidney transplantation has expanded the amount of information available to clinicians before deciding topursue biopsy. We evaluated how use of donor-derived cell-free DNA testing (dd-cfDNA) impacts histologic biopsy yield.
Methods: 1663 kidney transplant (KTx) recipients enrolled in the Kidney allograft Outcomes AlloSure Registry (KOAR, NCT03326076) were followed with dd-cfDNA post-transplant; testing was obtained either as part of a surveillance strategy or for-cause; indications for biopsy and histologic diagnoses were captured.This analysis included only for-cause biopsies.
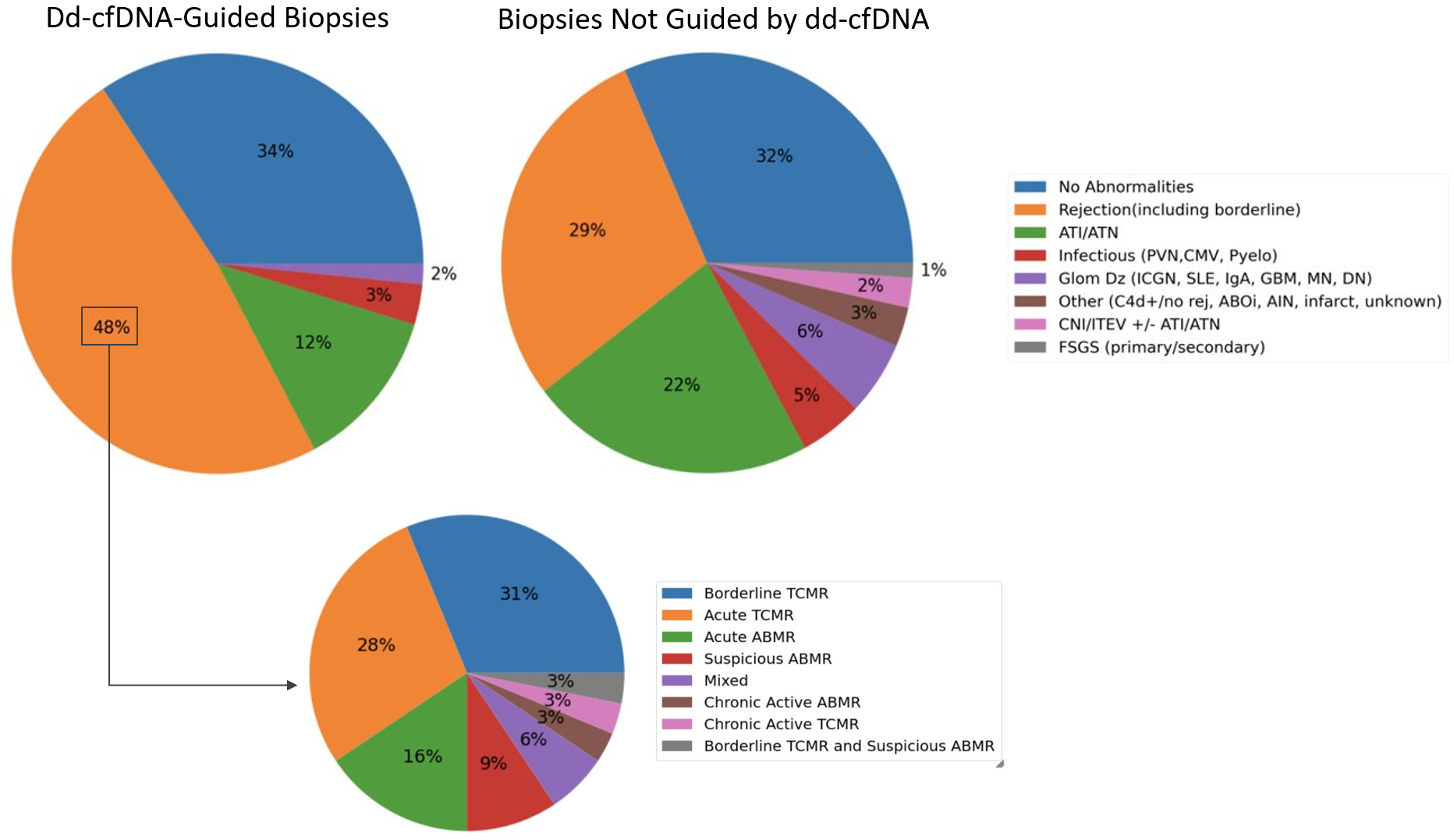
Results: 65 biopsies (from 59 pts) driven by elevated dd-cfDNA levels were compared to 540 biopsies (392 pts) obtained for causes other than elevated dd-cfDNA. Patient age (55 vs 56 years), percent deceased donor (84.75% vs 78.83%), and biopsy timing post-transplant (123 vs 105.5 days) did not differ betweengroups (Table 1).
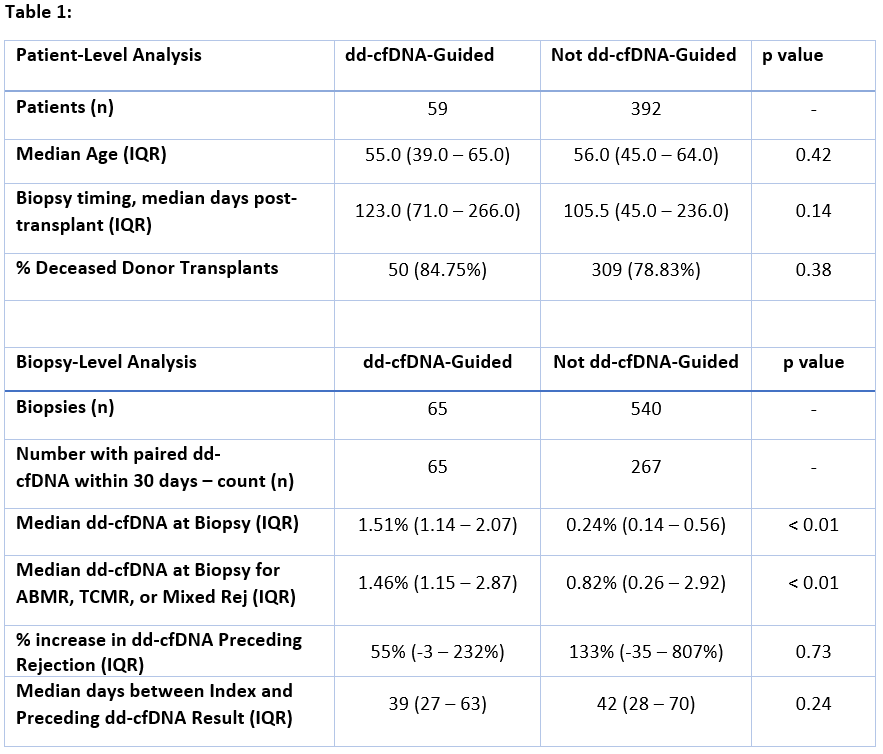
Among dd-cfDNA-guided biopsies, yield was enriched for rejection (48% vs 29%) and included fewer cases of ATI/ATN (12% vs 22%)compared to biopsies obtained for other causes (p<0.05) (Figure 1). Among dd-cfDNA-guided biopsies with rejection (ABMR, TCMR, or Mixed), median dd-cfDNA was 1.46% (IQR: 1.15 - 2.87). Among biopsies not performed due to elevated dd-cfDNA but with paired results available (n = 267), median dd-cfDNA was0.24%. Within this group, patients with rejection had median dd-cfDNA of 0.82% and demonstrated an increase of 133% from the preceding result.
Conclusions: Dd-cfDNA surveillance enhances the histologic yield for actionable results in for-cause allograft biopsies. A low dd-cfDNA result can obviate theneed for biopsy even in the presence of other clinical factors that are routinely used to guide the biopsy decision.

right-click to download
