Donor-derived cell-free DNA (dd-cfDNA) for assessment of response after treatment of allograft rejection
Reginald Gohh3, Matthew Weir1, Hannah Gilligan4, Vinaya Rao5, Muralidharan Jagadeesan8, Jeffrey Klein6, Nikhil Agrawal2, Grigory Shekhtman2, Yi Fu2, Enver Akalin7.
1Medicine, University of Maryland, Baltimore, MD, United States; 2Kidney Transplant, CareDx, Brisbane, CA, United States; 3Medicine, Alpert School of Medicine, Brown University, Providence, RI, United States; 4Medicine, Massachusetts General Hospital , Boston, MA, United States; 5Medicine, Medical University of South Carolina, Charleston, SC, United States; 6Medicine, University of Kansas, Kansas City, KS, United States; 7Medicine, Montefiore Medical Center, Bronx, NY, United States; 8Medicine, George Washington School of Medicine, Washington DC, DC, United States
Introduction: Surveillance of dd-cfDNA after therapy for rejection represents a promising strategy for monitoring post-treatment response. We assessed post-rejection kinetics of dd-cfDNA among kidney transplant recipients in the Kidney allograft Outcomes AlloSure Registry (KOAR, NCT03326076).
Methods: We identified patients with biopsy-proven allograft rejection (BPAR), defined as ABMR, TCMR, or Mixed (Banff 2019), biopsy-paired dd-cfDNA measurements (within ≤30d of biopsy), and follow-up results 60-150 days after BPAR. When available, treatment and follow-up histology data was also analyzed.
Results: 48 episodes of BPAR (24 TCMR, 19 ABMR, 5 Mixed) and paired dd-cfDNA results were identified in 42 patients (Table 1).
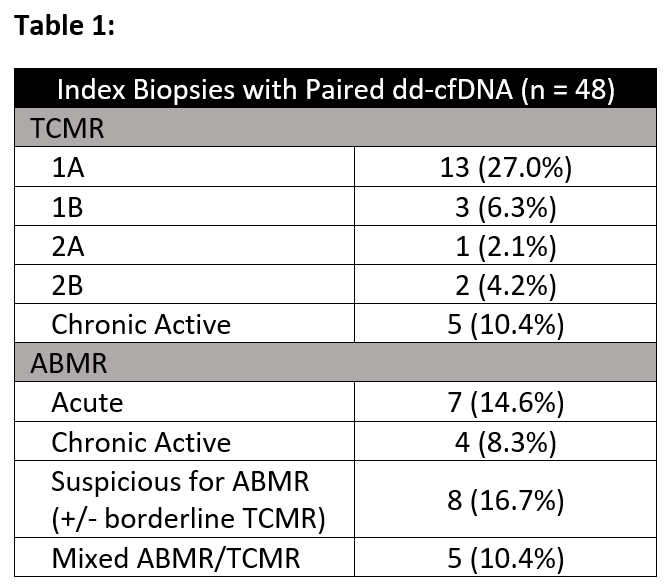
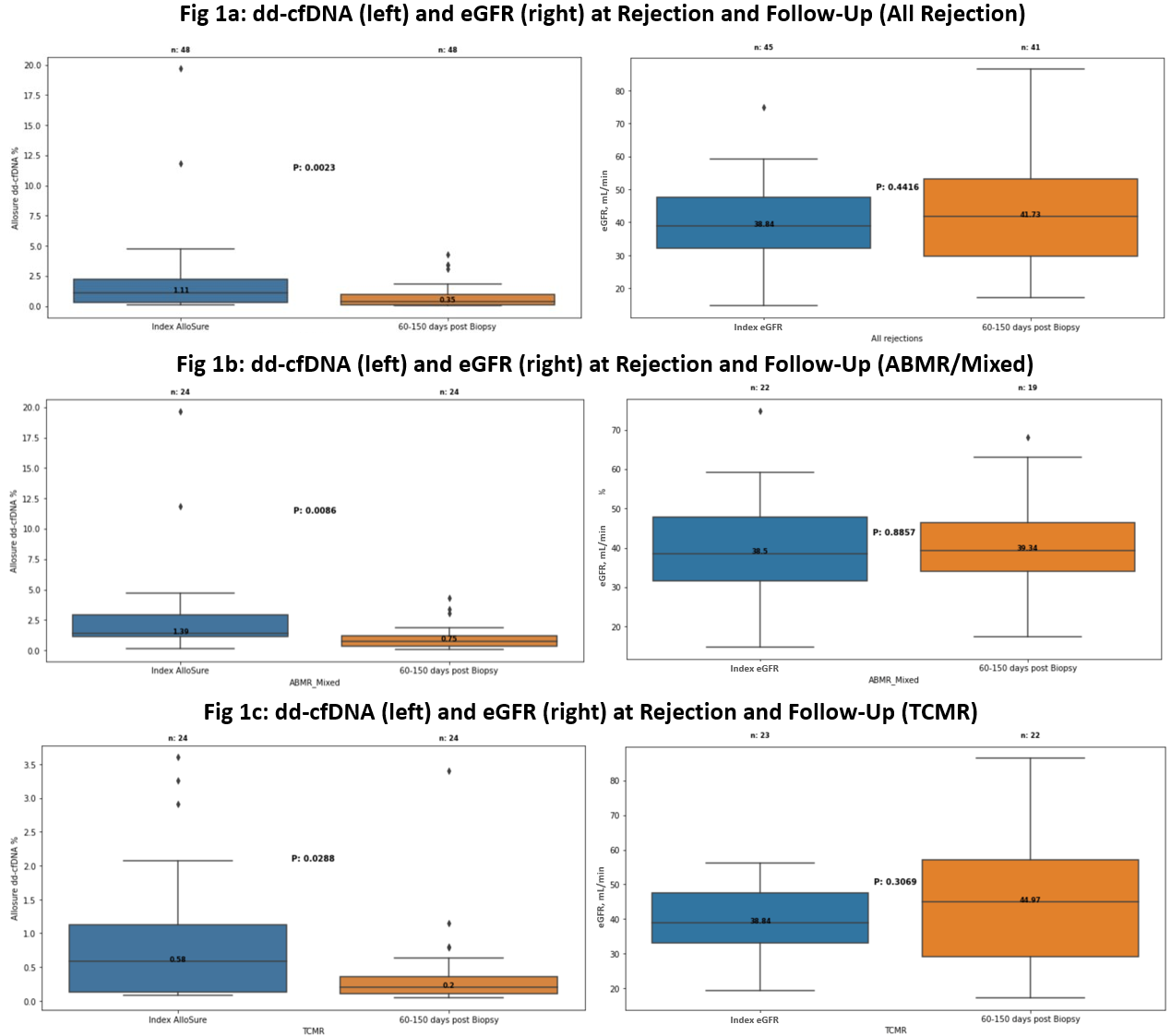
Overall, a significant reduction was seen between the median index (1.36%, IQR: 0.29-2.25) and follow-up dd-cfDNA results (0.35%, IQR: 0.13-0.95; p < 0.01) (Figure 1a). For patients with concurrent eGFR measurements, no statistically significant improvement was seen. These patterns held when analysis was limited to TCMR andABMR/Mixed rejections (Figure 1b, 1c). 7 patients (2 ABMR, 4 TCMR, and 1 Mixed; median index dd-cfDNA 1.15%, IQR: 0.31-2.46) had repeat biopsies within 30days of their follow-up testing; 1 patient with acute ABMR had chronic active ABMR on repeat biopsy, with a dd-cfDNA of 1.86%, while the remainder had eitherno rejection or borderline findings (median dd-cfDNA 0.74%, IQR: 0.30-2.59).
Conclusions: Our findings highlight that reduction in dd-cfDNA is commonly seen following BPAR, suggesting that most patients experience a “molecularresponse” to therapy. Studies are needed to better understand the prognostic significance of a molecular response and how persistent elevations in dd-cfDNAafter treatment should guide subsequent management.

right-click to download
