TUM012 – a new polymer for ex vivo allograft coating to minimize post-reperfusion thromboinflammation in kidney transplantation
Alireza Biglarnia1, Yuji Teramura3, Kristina Nilsson-Ekdahl2,4, Marianne Jensen-Waern5, Bo Nilsson2.
1Department of Transplantation, Clinical Sciences Malmö, Skåne University Hospital, Lund University, Malmö, Sweden; 2Department of Immunology, Rudbeck Laboratory, Uppsala University, Uppsala, Sweden; 3Department of Bioengineering, University of Tokyo, Tokyo, Japan; 4Linnæus Center of Biomaterials Chemistry, Linnæus University, Kalmar, Sweden; 5Department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden
Introduction: Ischemic reperfusion injury (IRI) can trigger a detrimental thromboinflammation that impacts post-transplant outcome. This response is often a result of direct interaction between effector molecules of the innate immune system and altered endothelial and epithelial cell surfaces of the allograft. TUM012, a polymeric construct of polyethylene glycol (PEG) conjugated to a lipid anchor, was developed for ex vivo graft administration to enable a stable and transient cell surface coating to reduce an IRI-induced thromboinflammation.
Method: Compiled selected data on two pre-clinical transplant studies are presented: 1) Six pigs underwent allogeneic en-bloc kidney transplantation. The en-bloc kidneys were retrieved from 6 donor-pigs after exposure to 3-5 min in situ warm ischemia followed by 24 hours cold ischemia. Pre-transplant, both kidneys within the en-bloc package were randomly assigned to FITC-conjugated TUM012 (at 2 mg/ml) and vehicle. Post-reperfusion consecutive blood (from graft vein) and tissue samples from both kidneys were taken during an observation period of 6 hours. 2) Ten kidney allografts from 5 donor pigs were exposed to in situ warm and cold ischemia (as study 1) and randomly assigned to 2 groups receiving either TUM012 (n= 6) or vehicle (n= 4). In both studies, TUM012 was given exclusively locally to preserved kidney allografts. Post-reperfusion, local consecutive blood (from transplant vein) and tissue samples were taken intraoperatively. Native bilateral nephrectomy was performed in all recipient animals. Systemic Blood sampling continued daily for a total observation period of 4 days. Biomarkers of thromboinflammation (C5b-9, C3a and thrombin-complex), cytokines release as well as serum creatinine were measured.
Results: Effective, non-toxic and transient coating on both endothelial and epithelial cells can be achieved by ex vivo administration of TUM012 to retrieved kidney allograft (fig 1a, b). This ex vivo coating was associated with a marked reduction of an instant post-reperfusion thromboinflammation (IPRT) that occurred within the graft (fig 1c). Furthermore, TUM012 effectively reduced systemic release of cytokines (fig 1d) as well as improved kidney function post-transplant.
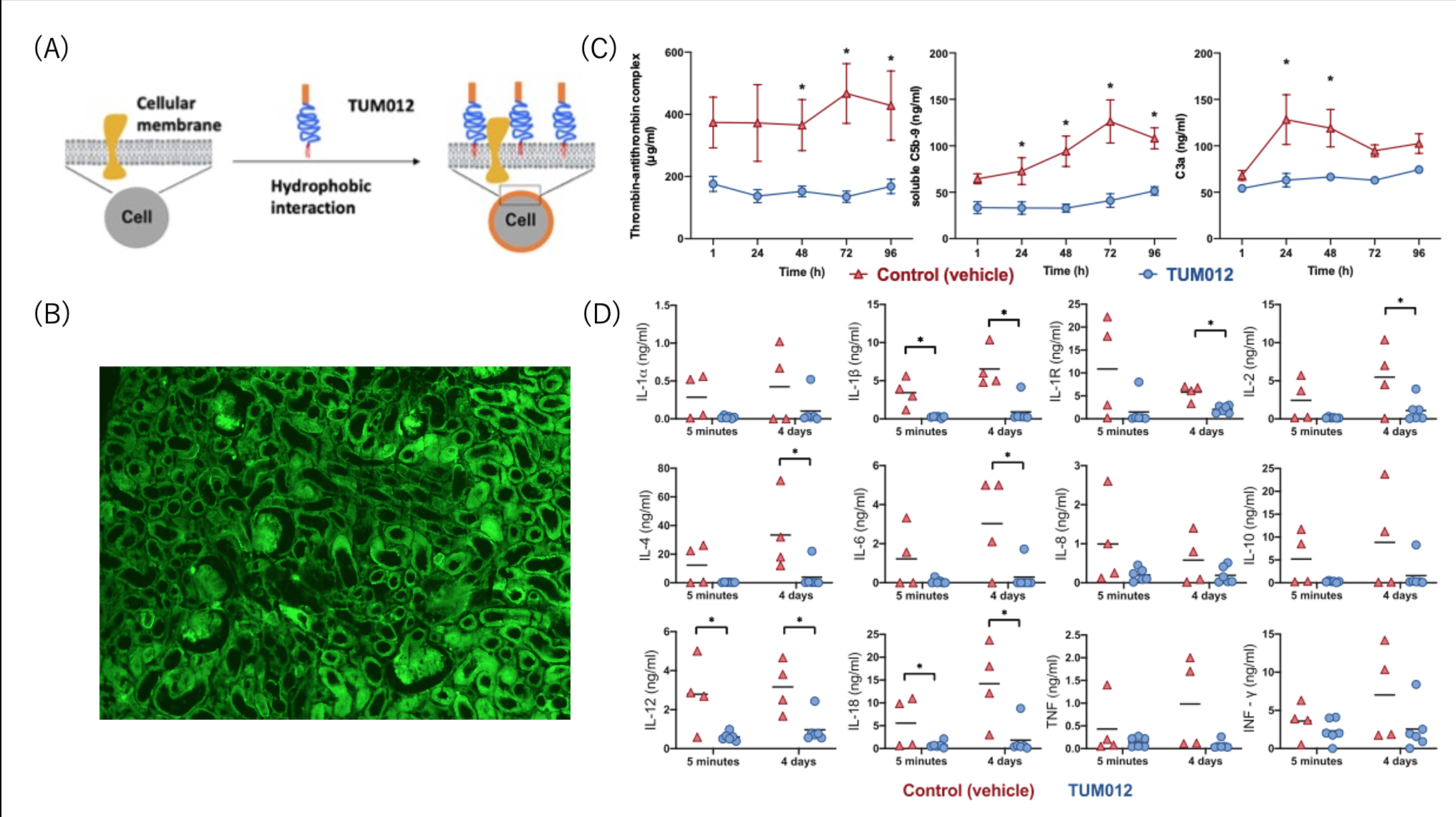
Conclusion: Here, we present selected data from an extensive pre-clinical assessment showing that ex vivo coating of kidney allografts with TUM012 seems to be safe and effective to reduce both the IPRT and the systemic cytokine release post-transplant. Consequently, this was assiciated with improved short-term kidney function. Currently, in a first-in-human study (ATMIRe), TUM012 is being evaluated for its safety and efficacy to reduce IPRT and to preserve kidney function in patients undergoing deceased-donor kidney transplantation at Skåne University Hospital in Malmö, Sweden.

right-click to download
