A phase 1, open-label evaluation of the pharmacokinetics and safety of a single dose of apraglutide in subjects with normal and impaired renal function
Gerard Greig1, Danni Cong2, Niamh Hurley1, Eric Michel1, Nader Youssef1.
1VectivBio, Basel, Switzerland; 2ICON PRA Health Sciences, Dublin, Ireland
Introduction: Patients with intestinal failure associated with short bowel syndrome (SBS-IF) have an increased risk of complications, including renal dysfunction. With currently available glucagon-like peptide 2 (GLP-2) therapy, dosage adjustments are recommended for patients with moderate and severe renal impairment and end-stage renal disease. Apraglutide has unique pharmacokinetic (PK) and pharmacodynamic (PD) properties, with preclinical studies indicating low clearance, slow absorption, and high protein binding resulting in a longer half-life than subcutaneously injected native GLP-2 and other GLP-2 analogs. Apraglutide is degraded into small peptides and amino acids via catabolic pathways, similar to endogenous GLP-2. Previous preclinical PK and metabolism trials showed no intact parent compound (apraglutide) was found in urine, suggesting renal elimination does not play a significant role in apraglutide removal.
Methods: This Phase 1, non-randomized, open-label, parallel-group trial evaluated the PK and tolerability of a single dose of subcutaneous apraglutide 5 mg in subjects with renal impairment and healthy volunteers matched for age and body weight. Part 1 assessed subjects with severe renal impairment (eGFR <30 mL/min/1.73 m2 not on hemodialysis) and subjects with normal renal function (eGFR ≥90). According to plan, the study was stopped after completion of Part 1 because exposure of subjects with severe renal impairment was not greater than that of healthy subjects.
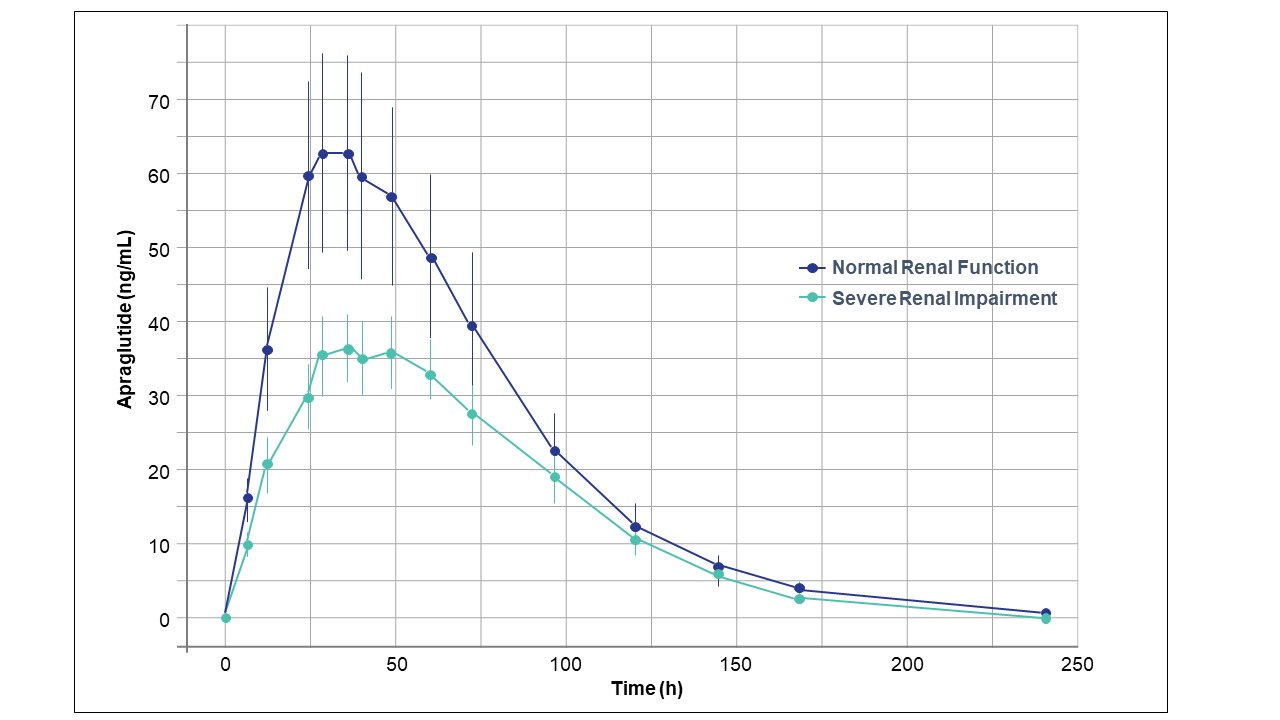
Results: Eight subjects with severe renal impairment and eight healthy matched subjects were enrolled and completed the study. There was no apraglutide overexposure in subjects with severe renal impairment compared to the healthy subjects. The geometric mean renal/healthy ratio was AUCinf (0.693) and Cmax (0.620). Three out of eight (37.5%) subjects with severe renal impairment reported 7 AEs, and 2/8 (25%) subjects with normal renal function reported 3 AEs. All AEs had resolved at the end of the study. The investigators considered 5 AEs related to apraglutide; all were reported by the subjects with severe renal impairment (1 increased alanine transaminase; 2 nausea and emesis; 1 erythematous papule; 1 ecchymosis at the injection site). The treatment-related AEs do not differ from those reported to date in other apraglutide clinical studies. No serious AE or death was reported. No clinically relevant changes within groups or differences between groups were observed over time in clinical chemistry, hematology, urine, or other safety parameters. No relevant changes in vital signs (blood pressure, heart rate, and body temperature) or ECGs occurred from screening to follow-up.
Conclusion: Exposure to apraglutide in subjects with severe renal impairment is not increased compared to healthy subjects. A single dose of 5-mg apraglutide in subjects with severe renal disease and healthy matched subjects was well-tolerated, and no unexpected safety issues were raised. The study results are clinically significant as 28% of patients with SBS-IF have renal impairment (Wang P et al. 2021) and may not be candidates for treatment with currently available GLP-2, which requires dose adjustment. By contrast, apraglutide dose adjustments are not necessary for subjects with renal impairment.

right-click to download
