The usefulness of non-HLA monitoring during graft rejection surveillance in heart transplantation
Karin Padros2, Olga L Vanco2, Maria Eugenia Fernandez2, Maria F Renedo3, Elian F Giordanino3, Liliana E Favaloro3, Rosario della Cella Figueredo3, Carolina B Putaro3, Roberto R Favaloro3, Daniel O Absi3, Carlos A Vigliano1, Ulises F Toscanini2, Alejandro M Bertolotti3.
1Anatomy Pathology, Fundacion Favaloro, Buenos Aires, Argentina; 2Histocompatibility, Fundacion Favaloro, Buenos Aires, Argentina; 3Intrathoracic Transplant, Fundacion Favaloro, Buenos Aires, Argentina
Introduction: The development of Donor Specific Antibodies against Human Leucocyte Antibodies (HLA-DSA) is one of the major causes of allograft dysfunction and rejection in solid organ transplants. However, many patients diagnosed with biopsy-proven Antibody-mediated rejection (AMR) do not have HLA-DSA. Many recent studies suggest that Non-HLA antibodies are involved in the immunopathogenesis of allograft rejection and poor graft survival. Anti-vimentin and myosin antibodies presence has been associated with AMR and anti-arginine, keratin, and tubulin antibodies with acute graft rejection. Therefore, performing continuous monitoring to detect the development of both anti-HLA and non-HLA DSA would be of paramount importance.
Aims:
Methods: Post-transplant 8 serum samples from 7 HTx recipients diagnosed with AMR by endomyocardial biopsy (EMB) and HLA- DSA absence in serum samples, were selected to be tested for Non-HLA antibodies. Nine HTx patients without rejection and HLA-DSA absence were also analyzed as a control group. The presence of DSA was determined by a single antigen bead assay using the Luminex platform (IMMUCOR) and interpreted with MATCH IT! Software. Non-HLA Antibodies were tested by Lifecodes Non-HLA Antibodies Kit (IMMUCOR) using Luminex platform and analyzed by LNHLA Analysis Tool. The AMR diagnosis was determined using the International Society for Heart and Lung Transplantation (ISHLT) grading scale updated in 2013. Biopsies were performed at the same time as the serum samples.
Results: The results of the Non-HLA antibody test of the patients who presented rejection in the EMB were compared with the control group that did not have biopsy-proven rejection results, evaluating the number of non-HLA antibodies detected in the study group compared to the control group.
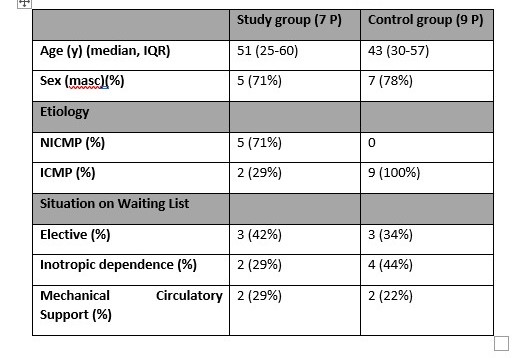
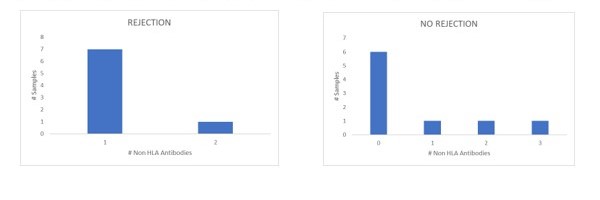
Conclusions: Although the number of patients analyzed in this study is reduced, we can observe that in all the patients who presented AMR without HLA-DSA, anti-Non HLA antibodies were detected, in comparison with the control group (without rejection), where the majority of the patients analyzed did not present antibodies. Regarding the Non-HLA antigens described more frequently associated with HTx rejection, we have not found this association in our group of patients, which we consider is due to the small number of samples in our study group. Our goal is to continue expanding the study group to extensively analyze our HTx patient population for non-HLA antibodies in the setting of rejection to determine the feasibility of using this assay as an additional tool in HTx graft rejection surveillance.

right-click to download
