Local immune cells' phenotype after corneal graft rejection
Justyna Sakowska1, Paulina Glasner2,3, Anna Rydz3, Anna Dukat-Mazurek1, Maciej Zieliński1, Piotr Trzonkowski1, Leopold Glasner3.
1Department of Medical Immunology, Medical University of Gdańsk, Gdańsk, Poland; 2Department of Anaesthesiology and Intensive Care, Medical University of Gdańsk, Gdańsk, Poland; 3Department of Ophthalmology, Medical University of Gdańsk, Gdańsk, Poland
Introduction: Penetrating keratoplasty is a vision-saving procedure. It is relatively easy to perform, as it does not require immunological matching, yet the grafts survival rates are high. The risk of graft failure increases in the case of repeated transplantation. The possible reason is that alloimmunization and subsequent immunological rejection prime the adaptive immune system for the next mismatched tissue. The rejection process, described generally on a mouse model, is mediated by CD4+ T cells, however, other populations of immune cells were also reported. This study aimed to characterize cells present in the rejected corneas of human patients qualified for retransplantation.
Methods: In the study, we analyzed cells isolated from explanted corneas from patients qualified for corneal transplantation (TX), either first procedure (primary TX, PTX group n=13) or retransplantation (repeated TX, RTX group n=13). The tissue was incubated for 24h in a culture medium at 37oC to allow immune cells to release from the tissue. For comparison, peripheral blood (PB) was acquired. The samples were analyzed by flow cytometry for lineage markers of T, B, and NK cells, T regulatory cells (Tregs), memory phenotype (CD62L, CD45RA), and Helios expression.
Results: Figure 1 presents representative cytograms of cells isolated from cornea tissue. The most abundant population of mononuclear cells in the cornea was T cells (median=54,4%), with a negligible level of B cells (Median=0,55%). Both CD4+ and CD8+ T cells were present in proportion comparable to peripheral blood, in both groups. There was a significantly more effector memory CD4+ (PTX Me=80,3%; RTX Me=61,9%; p=0,04) and CD8+ (PTX Me=83,3%; RTX Me=74,4%; NS) T cells within the tissue than in PB. The lower level of effector memory cells in RTX group was compensated with increase in central memory compartment. Tregs were detected in both groups in similar proportion to PB (Me=7,0% in cornea and Me=6,5% in PB). Tregs present in the cornea seems to have a low proportion of Helios positive cells with the median of Helios+ to Helios- Tregs 1:2. Interestingly, the PTX group showed a higher percentage of Helios+ Tregs in corneal infiltrate compared to the RTX group (p= 0,0254).
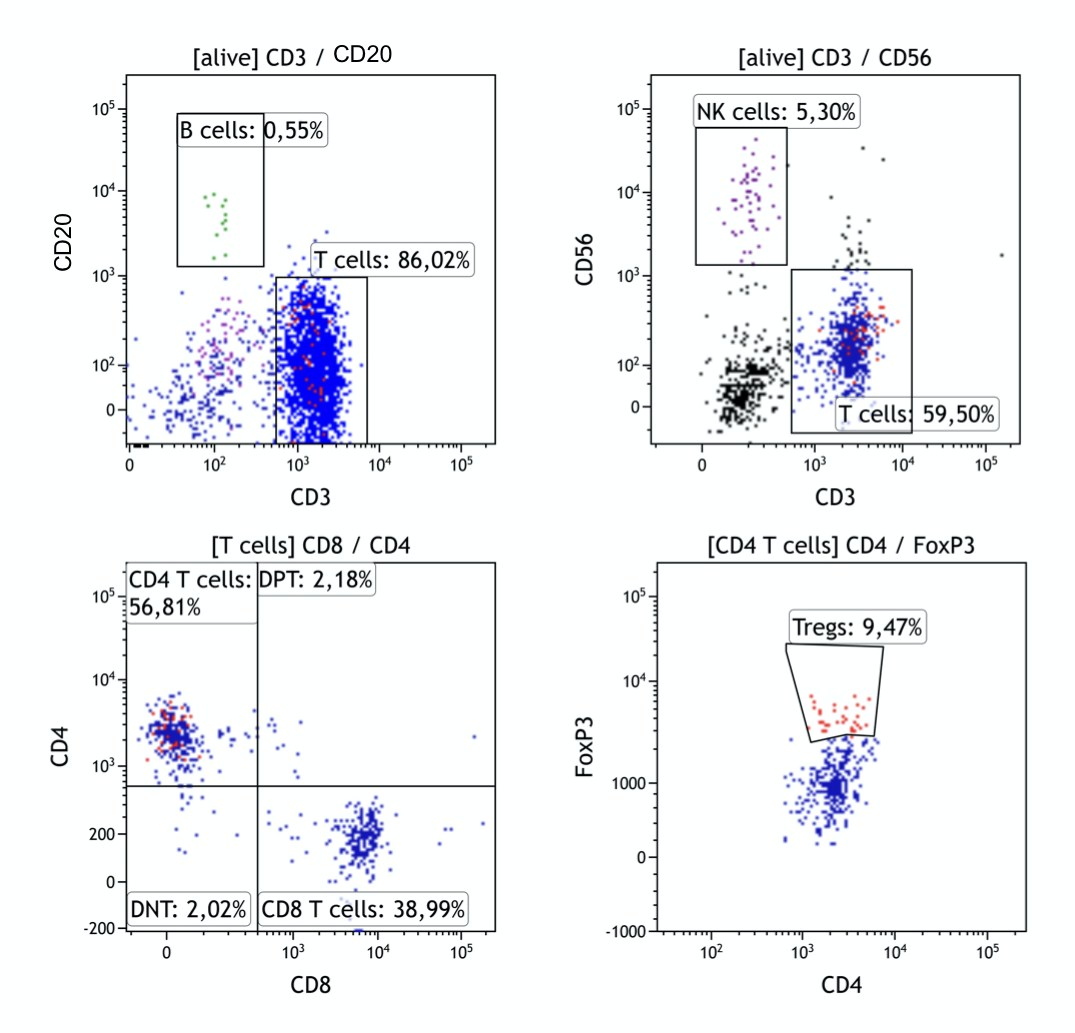
Conclusion: Corneas explanted from human patients present a mixed infiltration of immune cells. Both rejection process and primary corneal diseases facilitate immune cells infiltration into the tissue. T cells are generally the effector memory phenotype suggesting activation of the adaptive immune response, moreover, rejection of the allograft is associated with a higher proportion of central memory T cells. Within the tissue Tregs are present, however, low expression of Helios transcription factor suggests their instability. Understanding the immune status of primary corneal diseases may improve evaluation of allograft rejection risk while understanding the process of rejection will allow for a better management of transplant patients.
The study was supported by the project POWR.03.05.00-00-z082/18 co-financed by the European Union through the European Social Fund under the Operational Programme Knowledge Education Development 2014–2020.

right-click to download
