Dongkyu Han, Korea has been granted the TTS-KST International Transplantation Science Mentee-Mentor Awards
Mono-maintenance therapy with anti-ICAM-1 monoclonal antibody induced long-term liver allograft survival in nonhuman primates
Dongkyu Han1,2, Gwangmin Lee1,2, Joon Young Jang2, Suk Kyun Hong3, YoungRok Choi3, Jae-Il Lee1, Kyung-Suk Suh3, Nam-Joon Yi3, Jaeseok Yang2.
1Department of Medicine, Graduate School, Seoul National University College of Medicine, Seoul, Korea; 2Division of Nephrology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea; 3Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
The one study.
Introduction: MD-3 is an anti-human intercellular adhesion molecule-1 (ICAM-1) monoclonal antibody (mAb) that can induce tolerogenic myeloid cells and donor-specific T cell unresponsiveness rather than adhesion inhibition. In a previous study, we demonstrated that short-term (3 months) therapy of MD-3 effectively suppressed liver allograft rejection and thereby prolonged live allograft survival in nonhuman primate (NHP) models; however, liver allografts experienced chronic rejection later and subsequent graft failure. Based on these promising data, we aimed to investigate whether MD-3 can replace toxic calcineurin inhibitors as a maintenance therapy.
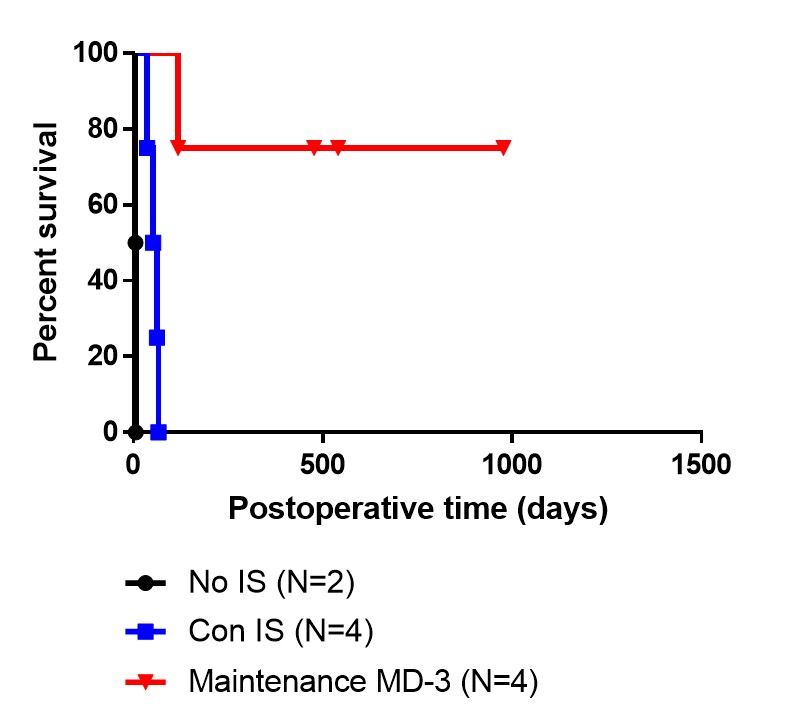
Methods: We used a rhesus macaque liver transplantation model and compared three groups - no immunosuppression group (n = 2), conventional immunosuppression group (n = 4), and MD-3 group (n = 4). Conventional immunosuppression consisted of 2 months of corticosteroid, 3 months of advagraf, and 4 months of mTOR inhibitor. MD-3-based immunosuppression consisted of conventional triple immunosuppression and 13 doses (8 mg/kg/dose) of MD3 for the initial three months, followed by monthly maintenance MD-3 dose after 3 months. Protocol liver biopsy was performed at 4 months, 12 months, 16 months, and 24 months after transplantation.
Results: No immunosuppression control group showed severe acute allograft rejection and showed very short survival (survival duration: day 5, day 6). Conventional immunosuppression group showed acute or chronic allograft rejection and all lost liver allograft by day 66 (survival duration: day 36, 52, 62, 66). One member in the MD-3 group lost liver allograft on day 118 due to liver cirrhosis that was related to hepatic venous obstruction. The other three members in the MD-3 group kept liver allograft well until now (day 501, 564, 998). One member in the MD-3 group showed mild abnormality in liver function test and mild acute T-cell-mediated rejection on protocol biopsies. The other two live members showed normal liver function test results and no evidence of rejection on protocol biopsy. When we checked MD-3 trough levels, MD-3 levels during the initial two months were lower in the member that experienced mild rejection, compared to the other members of MD-3 group.
Conclusions: Long-term MD-3 mono-maintenance therapy suppressed liver allograft rejection and kept liver allograft survival without maintenance of conventional immunosuppressants including calcineurin inhibitor. Therefore, MD-3 is a promising as a mono-maintenance therapy without calcineurin inhibitors for liver transplantation, although MD-3 failed to induced liver allograft tolerance as a short-term therapy.

right-click to download
