Impact of desensitization in immunological low-risk patients in kidney transplantation
Youngmin Ko1, Hye Eun Kwon1, Sung Shin1, Young Hoon Kim1, Hyunwook Kwon1.
1Department of Surgery, Asan medical center, Seoul, Korea
Background: Pretransplant DSA is considered to increase the risks of early rejection and long-term graft failure in kidney transplantation (KT). However, there is a lack of consensus on whether desensitization before transplantation is necessary for patients with low mean fluorescence intensity (MFI) value of donor specific antibody (DSA). In ABO-incompatible (ABOi) KT, recipients underwent pretransplant desensitization treatment, using rituximab, plasmapheresis (PP), or intravenous Ig (IVIG). This study aims to examine the efficacy of pretransplant desensitization in immunological low-risk patients by comparing the incidence of one year de novo(dn) DSA in ABOi and ABO compatible (ABOc) KT.
Methods: This retrospective observational study included pre-transplant DSA positive recipients with maximal DSA MFI <5000 and negative flow‐cytometric crossmatching (FCXM) between January 2014 and December 2019. A total of 274 patients were divided into an ABOc(n=222) and an ABOi (n=87) group.
Results: The baseline characteristics showed no difference between the two groups except the degrees of PRA class I and II were significantly higher in the ABOc group (P = 0.010 and P = 0.036). The incidence of dn DSA during one year after KT was significantly lower in ABOi group than in the ABOc (n=34, 15.3% vs. n=2, 3.8% P = 0.028). Multivariate analysis indicated that ABO incompatibility was the only significant factor associated with the development of one year de novo DSA (OR[CI], 0.22 [0.05 – 0.95]; P = 0.043). Overall rejection-free survival was inferior in patients who developed the dn DSA within one year than in patients without dn DSA for one year following KT (P < 0.001).
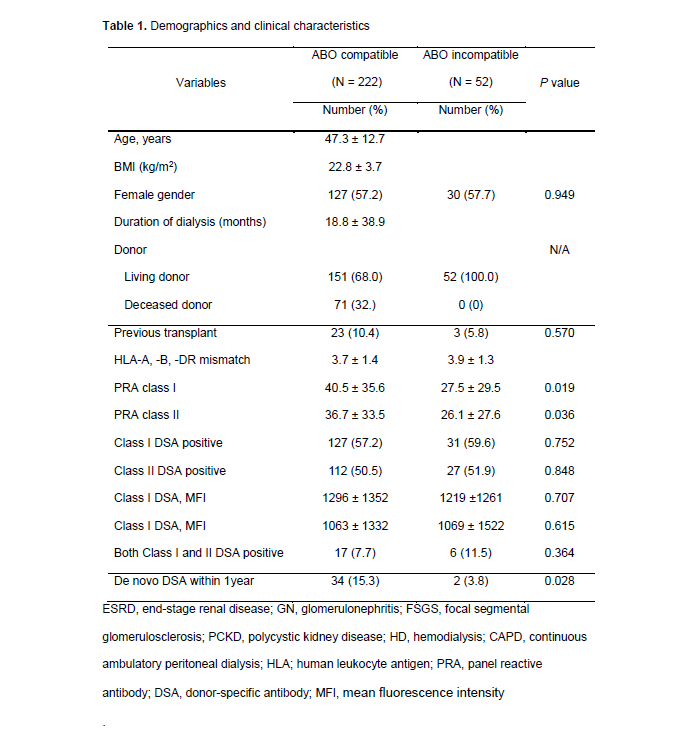
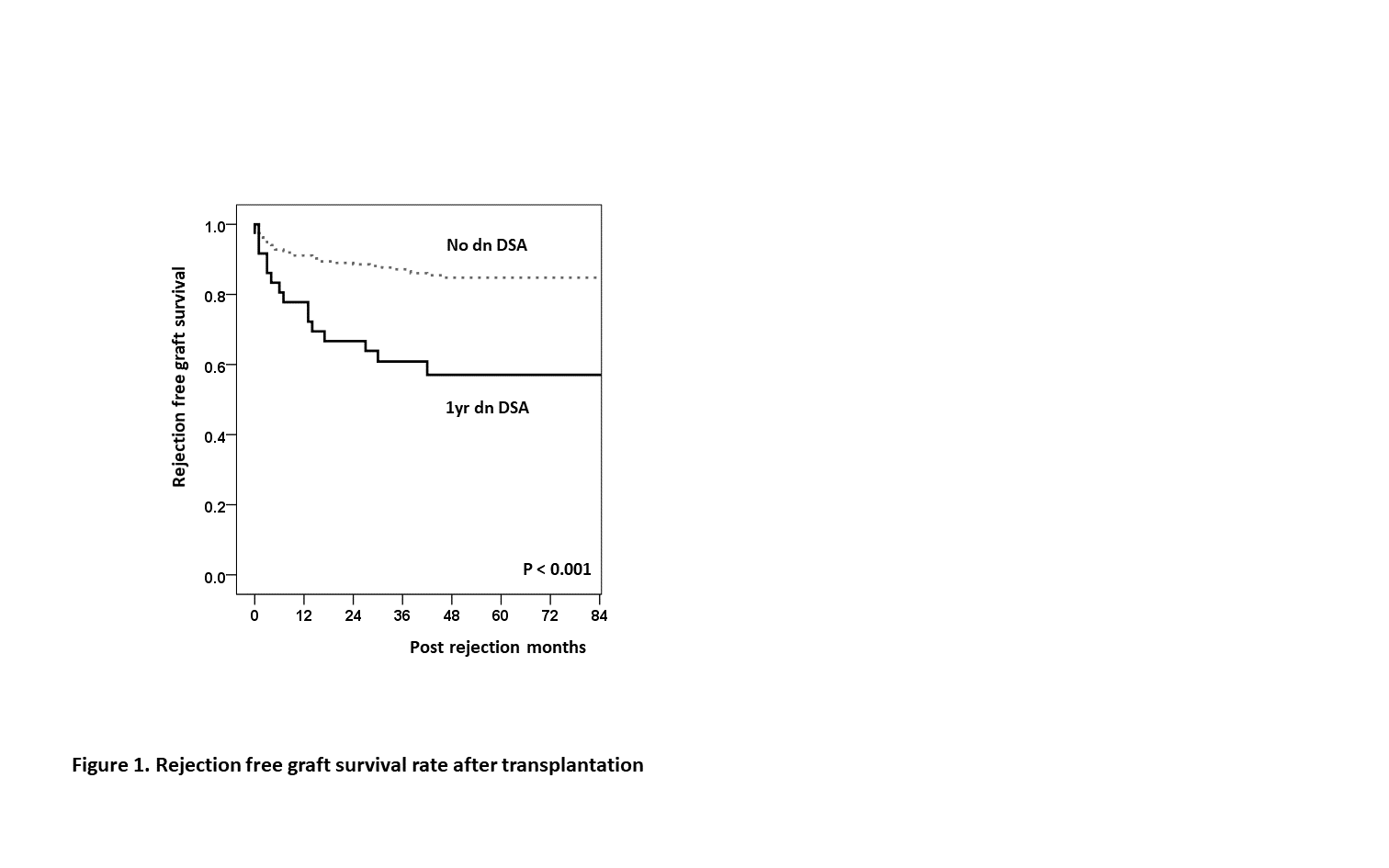
Conclusions: Pretransplant desensitization in patients with positive DSA and FCXM may have beneficial effects on the development of dn DSA. These results indicate the need for studies in larger patient cohorts, including randomized controlled trials, to examine the effectiveness of pretransplant desensitization in immunological low-risk patients in ABOc KT.

right-click to download
