A multi-institutional study of factors determining cardiac allograft function in children at 3 years post-transplant: absence of impact of donor specific antibodies
Steven A. Webber1, Hyunsook Chin2, Brian Armstrong2, Charles E Canter3, Anne I Dipchand4, Debra A Dodd1, Brian Feingold5, Jacqueline Lamour6, William Mahle7, Joseph W Rossano8, Tajinder P Singh9, Warren A Zuckerman10, Yvonne Morrison11, Helena Diop11, Carol Bentlejewski12, Jonah Odim11, Adriana Zeevi12, James Wilkinson1.
1Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, United States; 2Rho Federal Systems Division, Chapel Hill, NC, United States; 3Pediatrics, Washington University School of Medicine, St. Louis, MO, United States; 4Pediatrics, Hospital for Sick Children, Toronto, ON, Canada; 5Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; 6Pediatrics, Icahn School of Medicine at Mt. Sinai, New York , NY, United States; 7Pediatrics, Children's Healthcare of Atlanta, Atlanta, GA, United States; 8Pediatrics, Children's Hospital of Philadelphia, Philadelphia, PA, United States; 9Pediatrics, Boston Children's Hospital, Boston, MA, United States; 10Pediatrics, Columbia University Medical Center, New York, NY, United States; 11National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States; 12Pathology, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
CTOTC-09 Study Group.
Introduction: We previously showed that highly sensitized and non-sensitized pediatric heart transplant (PHTx) recipients had similar 1 year survival (CTOTC-04 study). In this follow-up study (CTOTC-09), we assess risk factors for impaired allograft function at 3 years post-Tx including the impact of ‘preformed’ (at Tx) and first year newly detected donor specific antibodies (ndDSA).
Methods: Consecutive children listed for Tx at 9 North American centers were prospectively enrolled. Management of sensitization, immunosuppression and rejection surveillance were standardized. The primary endpoint was pulmonary capillary wedge pressure (PCWP) at 3 years post-PHTx. Secondary endpoints included other hemodynamics measures, ejection fraction (EF), brain natriuretic peptide (BNP), graft and patient survival and rejection. DSA was defined as single antigen Luminex testing as ≥1 antibody specific to donor HLA antigens with MFI>1000 (based on core laboratory).
Results: Of 407 children enrolled, 370 achieved PHTx (mean age 8.7 years). Sensitization status at PHTx was as follows: non-sensitized (n=163, 44%), sensitized/no DSA (n= 115, 31%), sensitized/DSA+ (n=87, 24%); unknown (n=5 (1%) unknown. Baseline characteristics among these groups differed significantly by ethnicity (p=0.024), height (p=0.017), weight (p=0.017), ICU admission (p=0.009), and prior sensitizing events (p=0.027). At 3 years, subjects with vs. without early DSA (preformed and/or first year ndDSA) had comparable PCWP, EF, and BNP levels (Figure). There were also no significant differences between the 2 groups for other hemodynamic measures (pulmonary artery, right atrial, and left and right ventricular end diastolic pressures, cardiac index, EF, and BNP level (all P >0.05). For the 3 sensitization status groups at Tx, the presence/absence of first year ndDSA also did not influence any of these endpoints. Freedom from death or retransplant was not influenced by sensitization status at Tx, though freedom from rejection with hemodynamic compromise was inferior in those with one or more DSA at Tx > 4000 MFI. Freedom from acute antibody mediated rejection was lowest in the non-sensitized group (p<0.001). Multivariable analyses showed that higher PCWP at 3 years post-Tx was associated with 3-year age (p=0.017), 3-year weight (0.001) and coronary artery disease (CAD, p=0.035) but not with DSA status (preformed and/or first year ndDSA). Adverse graft function was associated with weight (Odds Ratio (OR) 1.03; 95% confidence interval (CI) 1.02, 1.05; p<001); and any rejection with hemodynamic compromise (OR 7.91; 95% CI 1.37, 45.75; p=0.021) but not with DSA status.
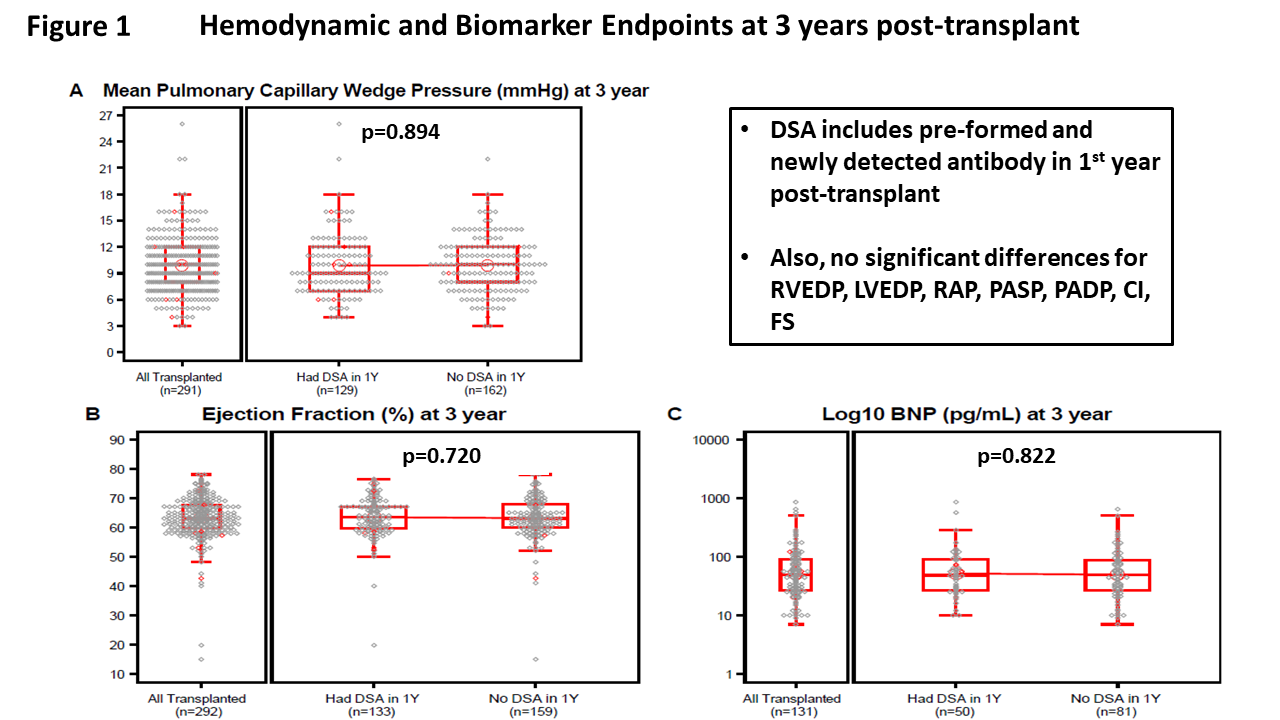
Conclusion: Sensitization status at PHTx and development of first year ndDSA are not associated with worse allograft function or inferior survival at 3 years. Ongoing follow-up, including assessment of CAD, continues. This information is critical for defining transplant strategies and the long-term impact of DSA on graft outcomes.
