Fast decrease of humoral response against SARS-CoV-2 in a kidney transplant cohort
Gisela Serra Rodrigues Costa1, Lara Judith Cabral Miranda2, Eric Arcanjo Bringel2, José Otto Reusing Junior3, Carina Ceneviva4, Aline Pivetta Cora4, Luciane Carvalho Sarahyba Silva4, Claudia Maria Meira Dias5, Patricia Alves Santos Udiloff5, Elias David-Neto3, Ligia Camera Pierrotti1,5.
1Departamento de Moléstias Infecciosas e Parasitárias, HC-FMUSP, São Paulo, Brazil; 2Faculdade de Medicina da Universidade de São Paulo, FMUSP, São Paulo, Brazil; 3Serviço de Transplante Renal , HC-FMUSP, São Paulo, Brazil; 4Divisão de Laboratório Central, HC-FMUSP, São Paulo, Brazil; 5Laboratório de Imunologia , DASA, São Paulo, Brazil
Introduction: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread from Wuhan, China since December 2019 and caused a large ongoing pandemic disease with high morbimortality. In Brazil the disease caused more than 22 million cases and 600 thousand of COVID-19 related deaths in the country at the end of the second year. Immunocompromised hosts such as kidney transplant recipients (KTR) were expected to have higher risk for severe disease and death due to their compromised T cell responses. There are few studies evaluating the immunological response and antibody dynamics to vaccine and natural infection stimulus in transplant recipients. The present study aims to estimate SARS-CoV-2 seroprevalence in KTR assisted at tertiary university hospital of São Paulo-Brazil through a longitudinal design.
Methods: This study is a prospective cohort of outpatient KTR based on four consecutive serological surveys plus clinical and epidemiological questionnaires. We present here the preliminary results from two completed surveys (performed from April to June-2021 and from Aug to Sept-2021, respectively). Presence of antibodies against SARS-CoV-2 was assessed using an electro-chemiluminescence immunoassay for qualitative detection of antibodies to SARS-CoV-2 nucleocapsid, the Elecsys Anti-SARS-CoV-2 from Roche (cut off index ≥ 1.0 = reactive, sensitivity of 99.5% and specificity of 99.8%). We considered effective vaccine doses if they have been administered at least 14 days before blood sample collection. We characterized three groups according to the number of effective vaccine doses administered between the first and second surveys: Group 0D, those who did not receive any dose; Group 1D, those who received 01 dose and Group 2D, those who received 02 dose between surveys.
Results: We recruited 170 participants; positivity rates in the first and second surveys were 20.1% (CI95% 14.2-26) and 23.2% (CI95%15.4-30.9). Only 95 (55%) participants completed both surveys with a median of 105 days (range 54-154) between surveys. Loss of follow-up were due to seven deaths related to COVID-19, one graft loss and ninety did not collect blood samples in time for the second survey. Confirmed COVID-19 was detected in 29 cases previously to survey 1 and in 6 cases between surveys 1 and 2. Excluding confirmed COVID-19 participants between surveys 1 and 2, the seroprevalence analysis showed an increase in seroprevalence rates as expected for Groups 1D and 2D but revealed an important decrease on the seroprevalence rates for Group 0D – from 13.6% (CI95% 2.9-35) to 9.1% (CI95% 1.1-29) between surveys.
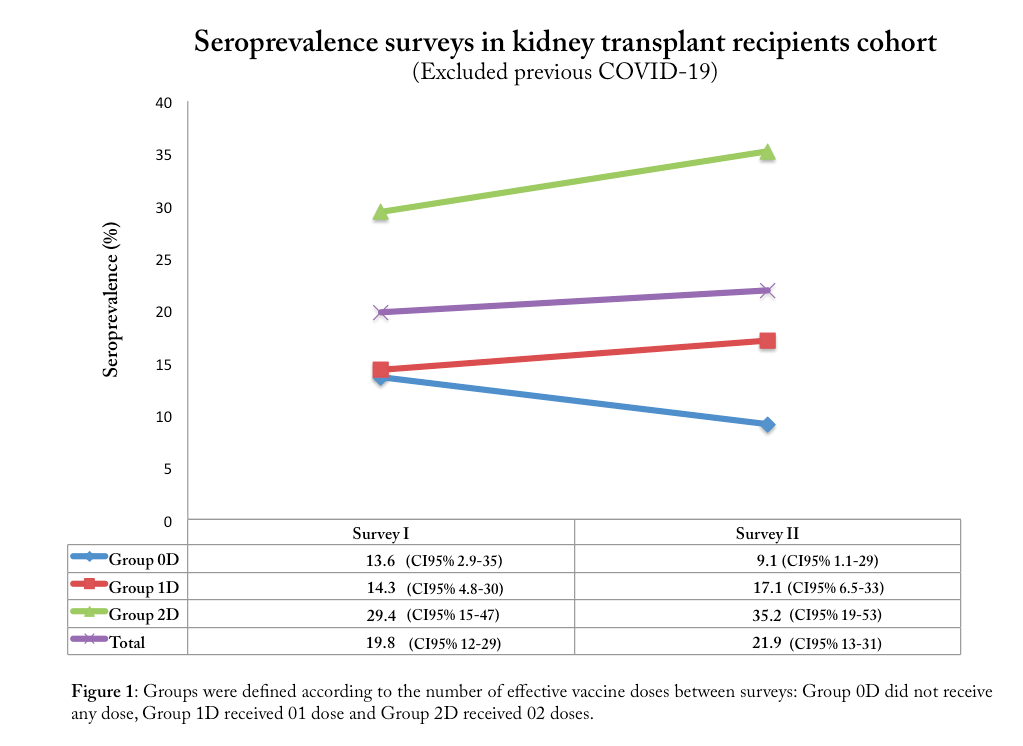
Conclusion: In this cohort of outpatient KTR of a tertiary hospital in São Paulo city, we highlight the decrease of seroprevalence rates between the surveys among those who were not exposed to any stimulus such as recent COVID-19 or vaccine. At the end of this survey we aimed to understand better the dynamic of antibodies in KTR.
