Extracorporeal membrane oxygenation use in orthotopic liver transplantation as a viable bridge to transplant and tool for intraoperative and postoperative rescue
Govind Rangrass1, Angelica Perez-Gutierrez2, Dinesh J Kurian1, Aalok K Kacha1, Kymberly Harrington1, John Fung2.
1Anesthesia and Critical Care, University of Chicago Hospital, Chicago, IL, United States; 2Transplant Institute, University of Chicago Pritzker School of Medicine, Chicago, IL, United States
Introduction: The use of extracorporeal membrane oxygenation (ECMO) to treat cardiac and pulmonary failure in orthotopic liver transplant (OLT) patients is uncommon and poorly understood despite the high morbidity and mortality related to these complications. We present one of the largest contemporary single-center case series of adult OLT patients requiring ECMO.
Methods: This is a retrospective study of adult patients undergoing OLT at University of Chicago from January 2017 to March 2022, who required ECMO before, during, or after transplant. We excluded pediatric patients and patients undergoing simultaneous heart-liver and heart-liver-kidney transplantation. We conducted a chart review to identify relevant patient and operative factors, length of hospital stay, postoperative complications, postoperative survival days, length of time on ECMO, and mortality.
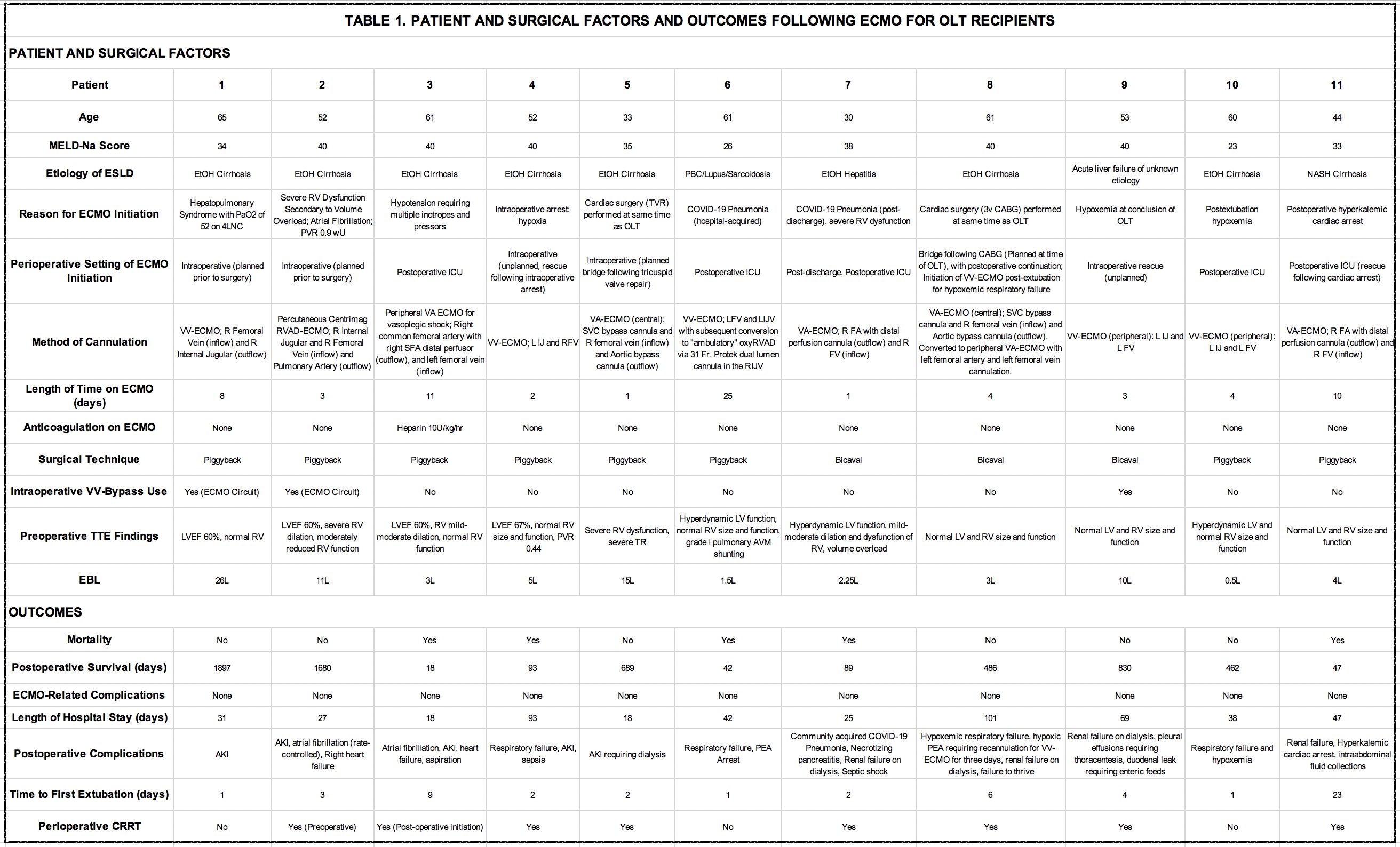
Results: A total of 273 patients underwent OLT during the study period. Eleven patients required ECMO, of which ECMO was initiated in 4 patients pre-transplant (planned), 2 patients intraoperatively (rescue), and 5 patients postoperatively (rescue). Patient and operative factors in addition to outcomes are described in Table 1. Six of the 11 patients survived to discharge following ECMO decannulation and all six are alive today. Three different types of ECMO were used: venoarterial (peripheral femoral venoarterial cannulation and central cannulation), venovenous ECMO (peripheral femoral venovenous cannulation), right ventricular assist device (RVAD) with an oxygenator (via a dual lumen cannula placed in the internal jugular so that its inflow lumen is in the SVC and outflow lumen is in the pulmonary artery).
Conclusions: ECMO may be used as a bridge to transplantation, for intraoperative rescue, and in the postoperative setting to provide mechanical support when medical management options for severe cardiopulmonary failure have been exhausted. Prior to using ECMO as a bridge to transplantation, evaluation should focus on the post-transplant reversibility of the condition requiring ECMO (e.g. advanced hepatopulmonary syndrome or severe right ventricular dysfunction). When ECMO is used for intraoperative rescue or postoperative decompensation, our outcomes are similar to previously published work. We noted improved outcomes when it is utilized for isolated hypoxemia without heart failure/vasoplegic shock. The exception to this observation was Patient 2 who had an RVAD/oxygenator placed prior to transplant for severe right ventricular dysfunction from volume overload with low pulmonary vascular resistance. In our experience, the need for rescue ECMO was unpredictable based on preoperative patient factors. Based on these results, we believe that ECMO is a viable strategy to provide additional support for patients with medically refractory but reversible hypoxemia or cardiogenic shock.