Outcomes after declining a donation after cardiac death (DCD) liver for transplant candidates in the United States
Tanveen Ishaque1, Mackenzie A Eagleson 1, Amber B Kernodle1, Mary G Bowring 1, Jennifer D Motter1, Yifan Yu1, Sile Yu1, Xun Luo1, Sommer Gentry2,3,4, Jacqueline M Garonzik-Wang 5, Elizabeth A King1, Dorry L Segev2,3,4, Allan B Massie2,3.
1Surgery, Johns Hopkins School of Medicine, Baltimore, MD, United States; 2New York University Langone Transplant Institute, New York, NY, United States; 3New York University Grossman School of Medicine, New York, NY, United States; 4Scientific Registry of Transplant Recipients, Minneapolis, MN, United States; 5University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
Introduction: In the context of the deceased-donor liver shortage, donation after cardiac death (DCD) provides an opportunity to expand the donor pool, but DCD organs confer higher risk of biliary complications and graft loss. Patients and clinicians who are offered a DCD liver must choose whether to accept the higher risk or remain on the waitlist in hopes of a better offer. We explored outcomes of candidates who declined a DCD offer, and investigated the survival benefit accepting vs declining the offer of a DCD liver. In addition, we estimated the number of additional liver transplants that could be achieved if DCD livers were utilized at the same rate as livers from otherwise-comparable DBD donors.
Methods: Using national registry data from the United States (SRTR) 2009-2020, we identified 1,564 candidates who accepted DCD offers ("acceptors") and 16,981 candidates who declined the same DCD offers ("decliners"). We characterized outcomes of decliners using a competing risk framework. In addition, we compared patient survival of acceptors vs. decliners using Cox regression. We used Poisson regression to compare observed discard of DCD livers to the number of discards expected based on donor characteristics other than DCD.
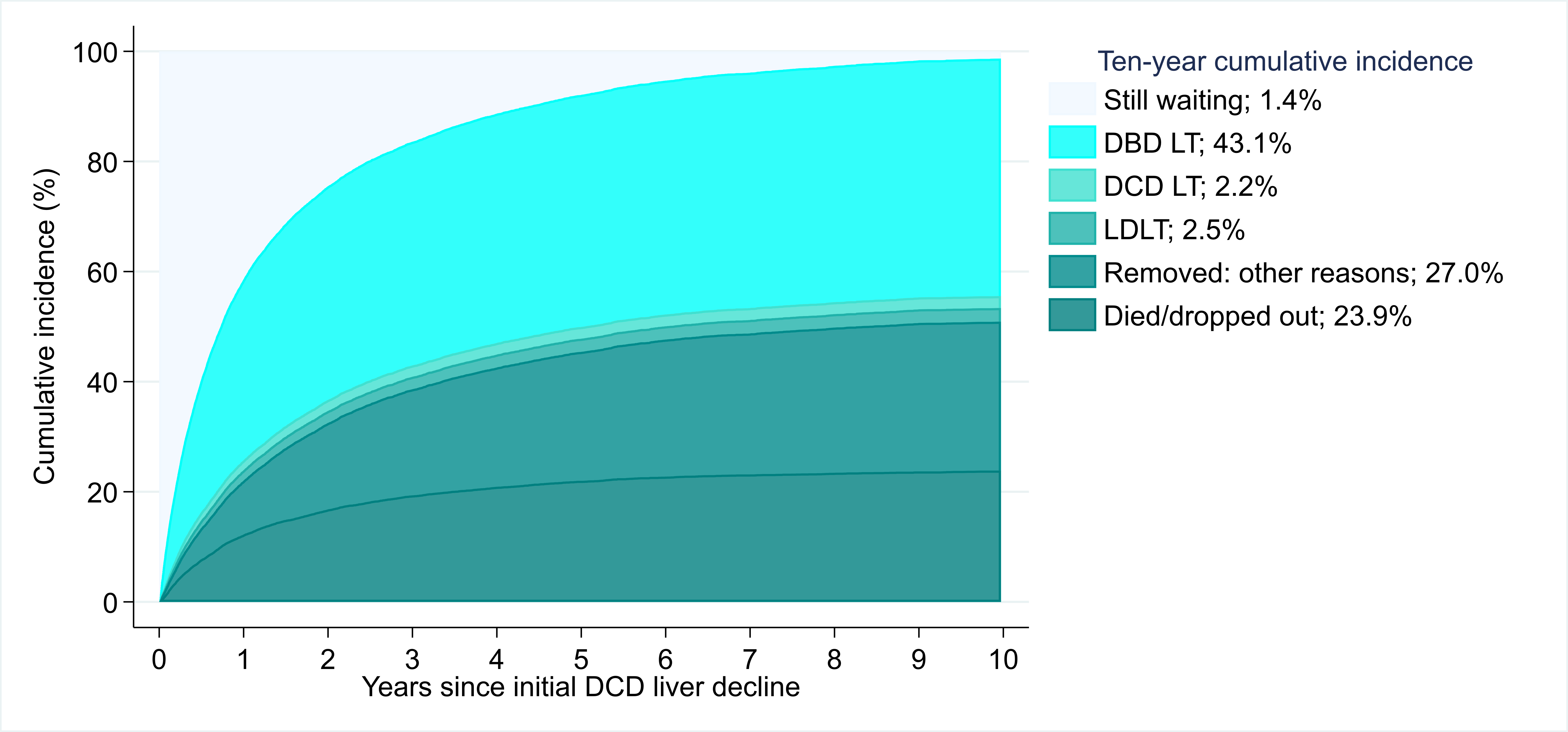
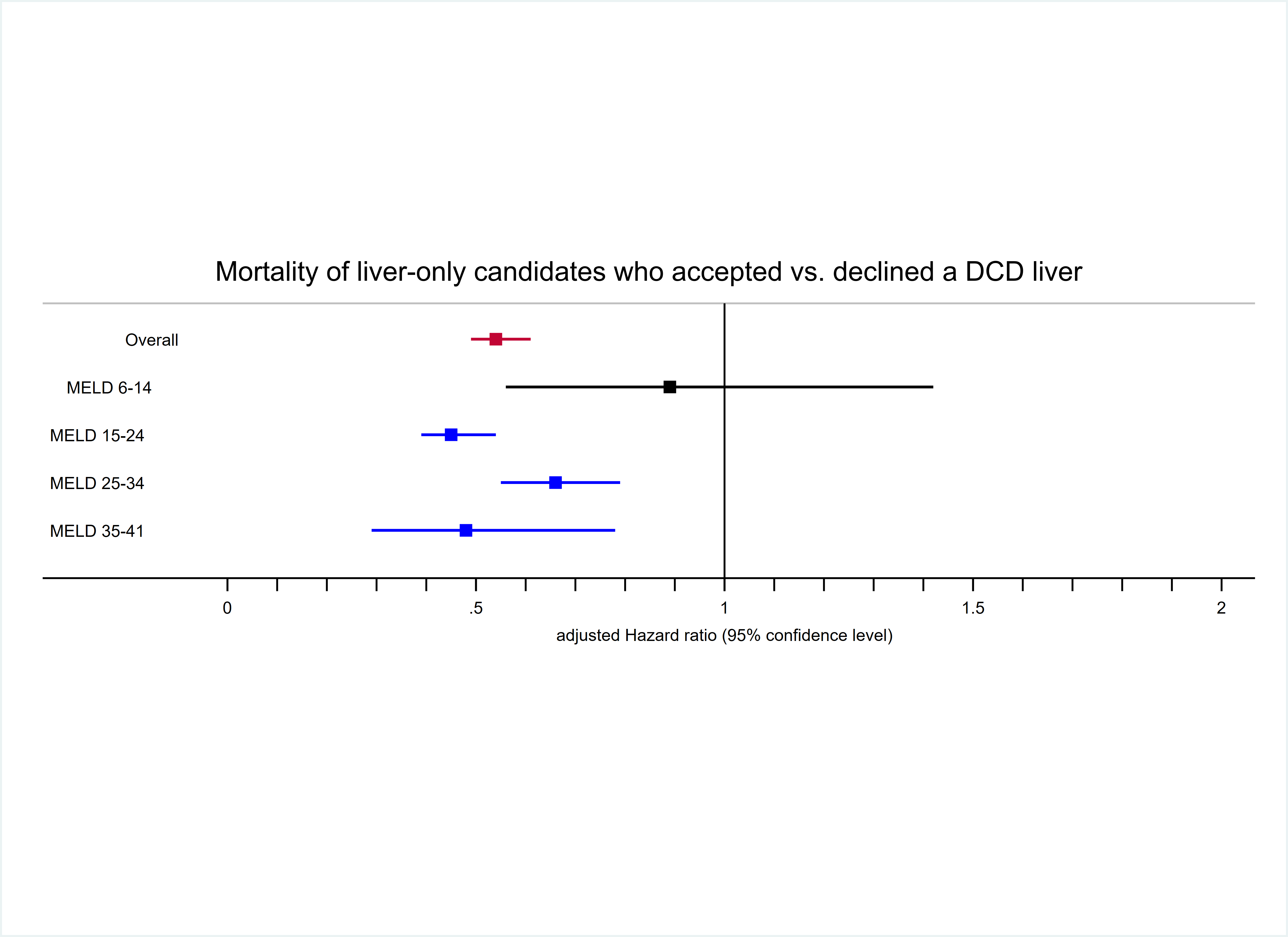
Results: Among DCD decliners, 50.9% died/dropped out from waitlist; only 43.1% were transplanted with a donation after brain death (DBD) liver. DCD acceptors had lower mortality compared to decliners at 10 years post-offer (35.4% vs. 48.9%, p<0.001) (Fiigure 1). After adjustment, DCD acceptors had 46% lower mortality risk compared to DCD decliners (aHR= 0.49 0.54 0.61). The survival benefit of DCD LT varied by MELD stratum. DCD acceptors and decliners with MELD 6-14 at offer had comparable risk of mortality (aHR= 0.56 0.89 1.42 , p=0.6 ). DCD offer acceptors had 55%, 34% and 52% lower risk of mortality compared to DCD offer decliners within MELD strata 15-24 (aHR= 0.39 0.45 0.54, p<0.001), 25-34 (aHR= 0.55 0.66 0.79 , p<0.001) and 35 or higher (aHR= 0.29 0.48 0.78 , p=0.004), respectively (Figure 2). Out of 1005 recovered DCD livers, 298 were discarded in 2019. If DCD livers were utilized at the same rate as comparable DBD livers, we estimated that 235 (78.9%) of them would have been transplanted.
Conclusions: Despite risk of biliary complications, DCD offer acceptance is associated with considerable long-term survival benefit for liver transplant candidates. Increased utilization of DCD livers should be encouraged.
This work was supported by grant number K01DK101677 (Massie), 5T32DK007713-23 (Eagleson), and K24DK101828 (Segev) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
