
Normothermic machine perfusion and orthotopic allotransplantation of the full length porcine intestine
Nader Abraham1, Elsa K. Ludwig2, Cecilia R. Schaaf2, Brittany Veerasammy2, Amy S. Stewart2, Caroline McKinney2, John Freund2, Kannan P. Samy1, Qimeng Gao1, Riley Kahan1, Katherine S. Garman1, Andrew S. Barbas1, Liara M. Gonzalez2, Debra L. Sudan1.
1Duke Ex-Vivo Organ Lab (DEVOL), Duke Division of Abdominal Transplant Surgery, Durham, NC, United States; 2Intestinal Regenerative Medicine Lab, North Caroline State University Department of Clinical Sciences, Raleigh, NC, United States
Duke Ex-Vivo Organ Lab (DEVOL). Intestinal Regenerative Medicine Lab.
Background: A limitation of intestinal transplantation is severe graft injury during cold storage, leadng to sepsis and rejection. Improved graft preservation will improve post-transplant outcomes. Recently, trials of oxygenated machine perfusion (MP) in liver transplantation show superior outcomes compared to cold storage. We hypothesized oxygenated MP of intestinal grafts would be feasible and improve outcomes after intestine transplantation, similar to other transplanted organs. The aim of this study was to develop a translational normothermic machine perfusion (NMP) protocol of full-length intestine allograft and validate feasibility of MP by orthotopic transplantation in a porcine model.
Methods: The NMP protocol underwent 3 iterative stages of development, generation 1, 2, and 3 (GEN1, GEN2, GEN3). GEN1 (n=8 grafts) protocol was adapted from published liver NMP protocols. Changes were made after review of 6-8 graft perfusions with a standardized approach. The perfusion circuit consisted of a graft chamber with open venous return into a reservoir below. A roller pump circulated perfusate from the reservoir into the oxygenator into the craniomesenteric artery at a mean arterial pressure of 50 ± 5 mmHg for 6 hours. Vasodilators were administered into the arterial line by constant infusion. Perfusion pressure, temperature, and arterial flow were monitored continuously using in-line sensors. A dialysis circuit was used to maintain the normal chemistries in GEN2 & 3. We compared gross and histologic appearance of paired samples from the time of organ procurement and after six hours of oxygenated MP. After optimization, transplantation of porcine intestine allografts after 6 hr NMP were then undertaken and postoperative recovery of gut function, physical activity, lab parameters and vital signs were monitored for 2 days before sacrifice.
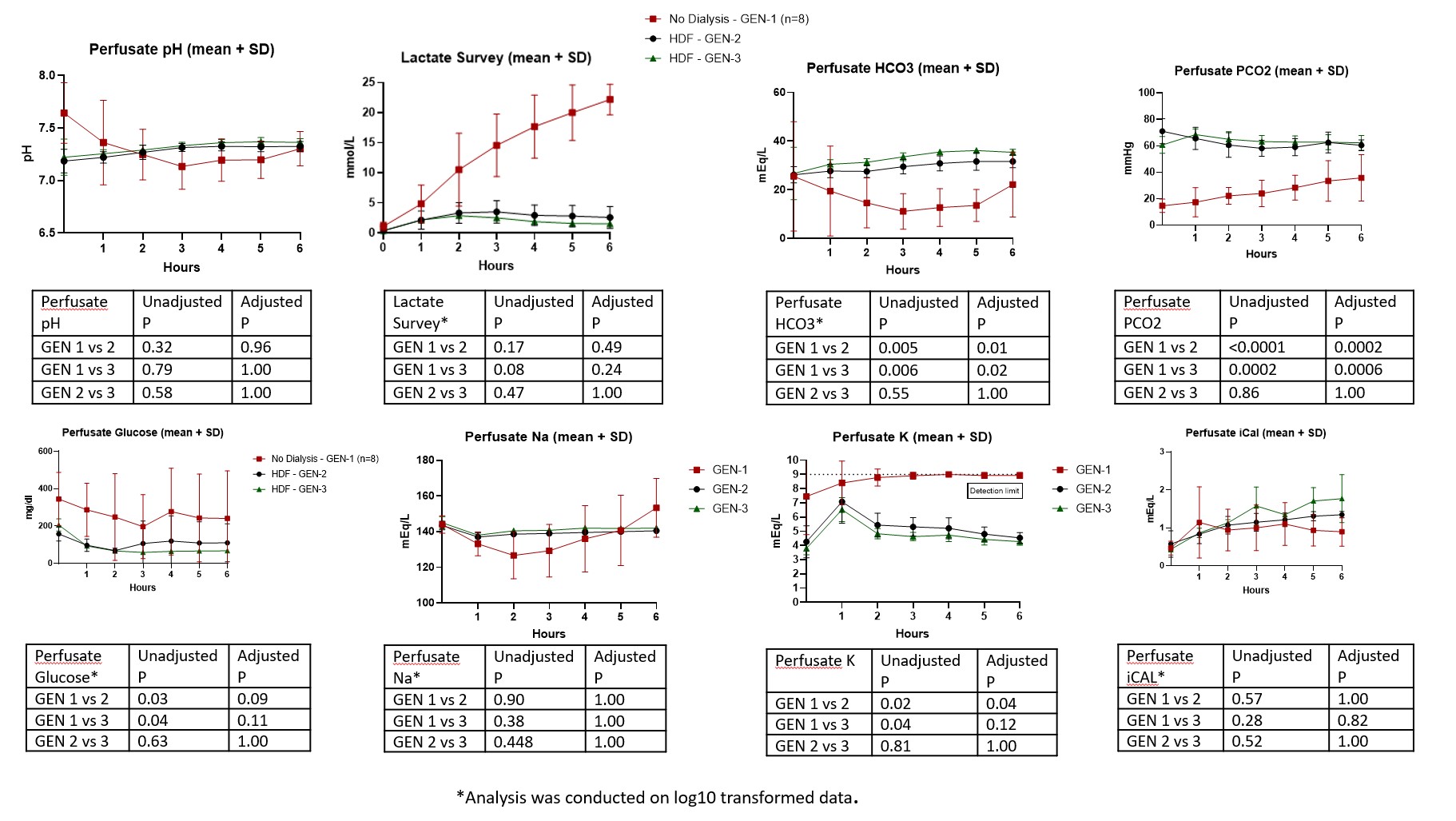
Results: During protocol development, we identified several factors that appear unique to the intestine allograft and posed challenges during MP, including metabolic, electrolyte, acid-base disturbances, as well as differential perfusion of the jejunum and Ileum. These factors coincided with graft and mesenteric edema, luminal hemorrhage, and ileal ischemia with the initial protocol. Addition of dialysis and introduction of vasodilating medications corrected the metabolic derangements in perfusate chemistries, and gross and histologic appearance suggested excellent preservation in GEN3. We report successful transplantation of 3 porcine intestinal allografts after MP with excellent post-operative recovery of gut function, physical activity, oral intake and maintenance of normal vital signs and lab values. At 48 hours inspection of the bowel graft demonstrated viable pink serosa without evidence of mucosal injury.
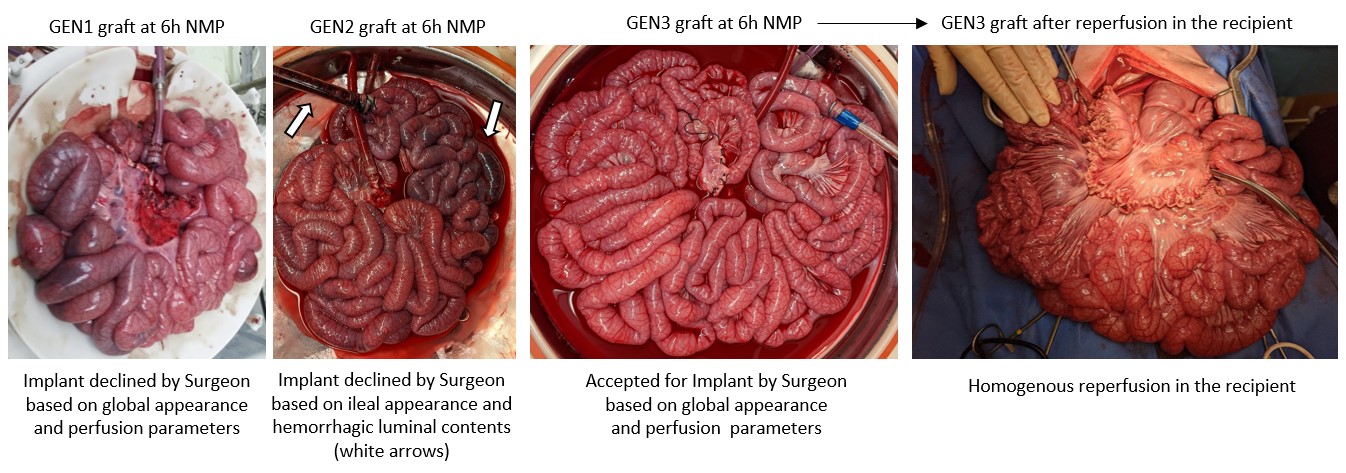
Conclusions: This study reports development and optimization of machine perfusion preservation of small intestine and successful transplantation of the intestinal allograft in a porcine model.
U.S Department of Defense (DOD).
