Longitudinal monitoring of pancreas and kidney transplant recipients using donor-derived cell-free DNA
Nicole Ali1, Zoe Stewart1, Jake Miles2.
1Transplant Institute, NYU Langone Health, New York, NY, United States; 2CareDx, CareDx, Brisbane, CA, United States
Introduction: Donor-derived cell-free DNA (dd-cfDNA) is validated for allograft surveillance in kidney transplant recipients; however, the utility in patients undergoing pancreas and kidney (PTx) transplantation has not been well studied. Pancreatic rejection remains a major clinical concern, yet diagnosis relies on pancreatic biopsy which is associated with significant risk of procedural complications. Early identification of allograft injury is imperative to optimize treatment and intervention. Here, we describe dd-cfDNA levels in PTx transplant recipients in the setting of clinical stability and immunological events.
Methods: PTx recipients were monitored longitudinally with dd-cfDNA (AlloSure, CareDx) at the discretion of the treating physician. dd-cfDNA was collected in tandem with standard of care laboratory testing including serum creatinine, amylase, lipase, DSA and BK PCR. Pancreatic graft dysfunction was defined by elevations in serum amylase/lipase or dysregulated glucose control. The reference population was defined by stable allograft function in the absence of immunological or clinical events. Immunological events were defined as clinically suspected allograft rejection or documented subtherapeutic immunosuppression.
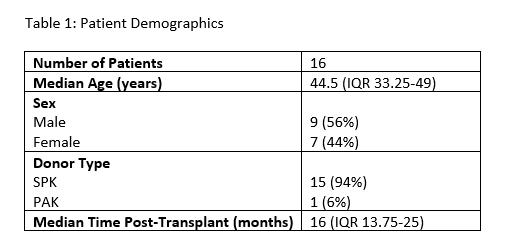
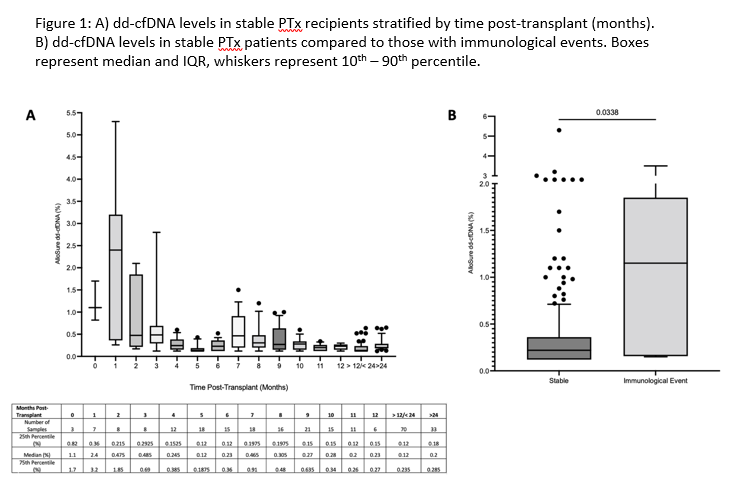
Results: A total of 16 PTx patients were monitored longitudinally with dd-cfDNA, including 15 SPK recipients and 1 pancreas after kidney transplant (Table 1). 12 patients met criteria for the stable reference population with a median dd-cfDNA level of 0.22% (IQR 0.12 – 0.36%, n=248). There was a trend towards increased variability in dd-cfDNA levels in the first 2 months post-transplant, after which there was no significant difference in dd-cfDNA stratified by time (Figure 1A). 4 patients developed 6 independent immunological events, which were associated with a significantly increased median dd-cfDNA level of 1.15% (IQR 0.15 – 1.85%) compared to 0.22% in the reference population (p = 0.03, Figure 1B).
Conclusions: In stable PTx recipients, dd-cfDNA levels remain low post-transplant and reflect the reference ranges validated in kidney transplant alone. Early variability in dd-cfDNA levels may be explained by recovery from ischemia reperfusion injury. Significant increases in dd-cfDNA were associated with immunological events including clinically suspected allograft rejection, highlighting its utility in longitudinal graft surveillance.