Development and evaluation of a cell therapy for ex vivo porcine kidney graft conditioning using exosomes derived from porcine urine progenitor cells
Perrine Burdeyron1,2, Sébastien Giraud1, Sonia Brishoual1, Estelle Lemarie1,3, Maïté Jacquard 1,3, Virginie Ameteau1,2, Nadège Boildieu 1,2, Hanno Maassen 4, Tobias M Huijink 4, Luc Pellerin1,2,3, Henri Leuvenink 4, Thierry Hauet1,2,3,5, Clara steichen1,2.
1INSERM , UMR-1313 IRMETIST, Poitiers, F-86021, France; 2Université de Poitiers, Faculté de Médecine et de Pharmacie, Poitiers, F-86073, France; 3CHU de Poitiers, Service de Biochimie, Poitiers, F-86021, France; 4Department of Surgery, Surgical Research Laboratory, University Medical Center Groningen, University of Groningen, Groningen, Netherlands; 5FHU SUPORT 'SUrvival oPtimization in ORgan Transplantation', Poitiers, F-86021, France
Introduction: Kidneys from extended criteria donors are particularly sensitive to ischemia/reperfusion injury. Among strategies to improve graft conditions, our study aims to test a cell therapy based on exosomes derived from porcine Urine Progenitor Cells (pUPCs) combined with Hypothermic Machine Perfusion (HMP) followed by Ex Vivo Normothermic Perfusion (EVNP).
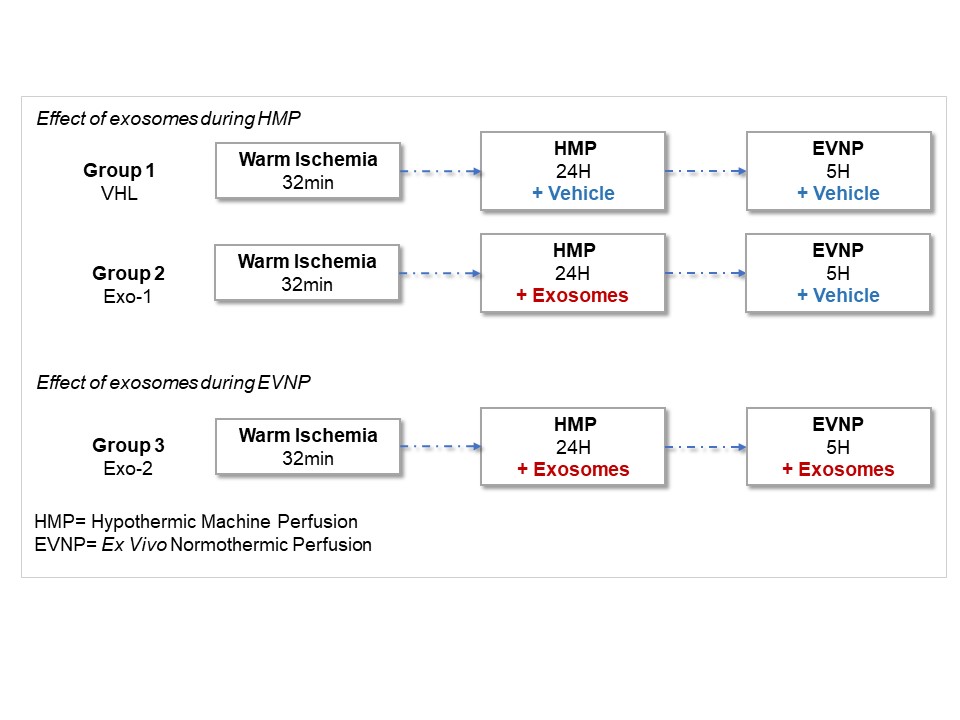
Materials and Methods: pUPCs were isolated and characterized by flow cytometry, immunofluorescence and western blotting. pUPC-derived exosomes were extracted by ultracentrifugation and characterized by western blotting, electronic microscopy and Nanosight Tracking Analysis. Porcine kidneys obtained from slaughterhouse were submitted to 32 min of warm ischemia then preserved by HMP for 24 hours at 4°C. Afterwards, kidney early function recovery was assessed using EVNP for 5 hours at 37°C. For cell therapy, pUPC-derived exosomes were injected via the porcine kidney artery. Three experimental groups were planned (n=6/group): Group 1: HMP/vehicle + EVNP/vehicle, Group 2: HMP/ pUPC-derived exosomes + EVNP/vehicle, Group 3: HMP/ pUPC-derived exosomes + EVNP-/ pUPC-derived exosomes. Analyses include functional parameters, histology, biomarkers, protein/cytokine and gene expression analyses in tissue samples and HMP and EVNP perfusates; as well as perfusate effect on in vitro porcine aortic cell cultures.
Results: Firstly, pUPCs were successfully isolated and amplified from porcine urine samples. Isolated pUPCs expressed stem cell markers such as SSEA4 embryonic stem cell marker, CD90 mesenchymal stem cell marker and are negative for CD45 hematopoietic stem cell marker and CD31 endothelial marker. Cells expressed kidney channel water markers such as AQP1 and AQP2 and can gain epithelial phenotype when differentiated into tubular cells. pUPC-derived exosomes expressed Alix and TSG101 exosomal markers and were of expected size (40-100nm of diameter) and delimited with a double membrane. Impact of exosome treatment during HMP and EVNP protocol is currently assessed. Preliminary results seem to show that pUPC-exosome injection seems feasible and does not induce acute kidney dysfunction. The potential beneficial effect will be assessed soon after the inclusion of the 18 porcine kidneys (13 performed for now).
Conclusion: This study is showing the feasibility to use porcine urine stem cells derived exosomes for renal graft conditioning, and will assess the impact of this therapy on kidney graft quality and early function.
Keywords: Kidney, Exosomes, Hypothermic machine perfusion, Ex vivo Normothermie perfusion, Stem cells, Cell therapy.
INSERM UMR-1313 IRMETIST, Poitiers, F-86021, France. Université de Poitiers, Faculté de Médecine et de Pharmacie, Poitiers, F-86073, France.
