Strategies to achieve long-term insulin independence using a multimodal approach to beta-cell replacement in patients with type I diabetes
Steven Wisel1, Miguel Nunez2, James M Gardner2, Giulia Worner2, Garrett R Roll2, Shareef M Syed2, Gregory Szot2, Medhi Tokhol2, Kristina Johnson2, Umesh Masharani2, Andrew M Posselt2, Peter G Stock2.
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA, United States; 2Division of Transplantation, University of California, San Francisco, San Francisco, CA, United States
Purpose: Beta cell replacement, via islet or solid organ pancreas transplant, remains the best strategy to restore insulin independence in patients with Type 1 diabetes mellitus (T1DM). Insulin independence beyond ten years is limited, with calcineurin inhibitors (CNI) contributing to beta cell toxicity and impairing renal function. Here, we report long-term results for multimodal beta cell replacement including islet and pancreas after islet (PAI) transplant with CNI-free immunosuppression (IS) regimens based on Belatacept (BELA) and Efalizumab (EFA).
Methods: Ten non-uremic patients with severe T1DM received their first islet cell transplant between 2007 and 2009 with CNI-sparing IS based on BELA (n=5) or EFA (n=5). Patients were monitored for a mean of 12.9 ± 1.1 years from islet transplant for beta cell function and renal function. Following islet failure, patients were considered for repeat islet infusion and/or PAI transplant. Circulating immunologic phenotype was characterized in the first year.
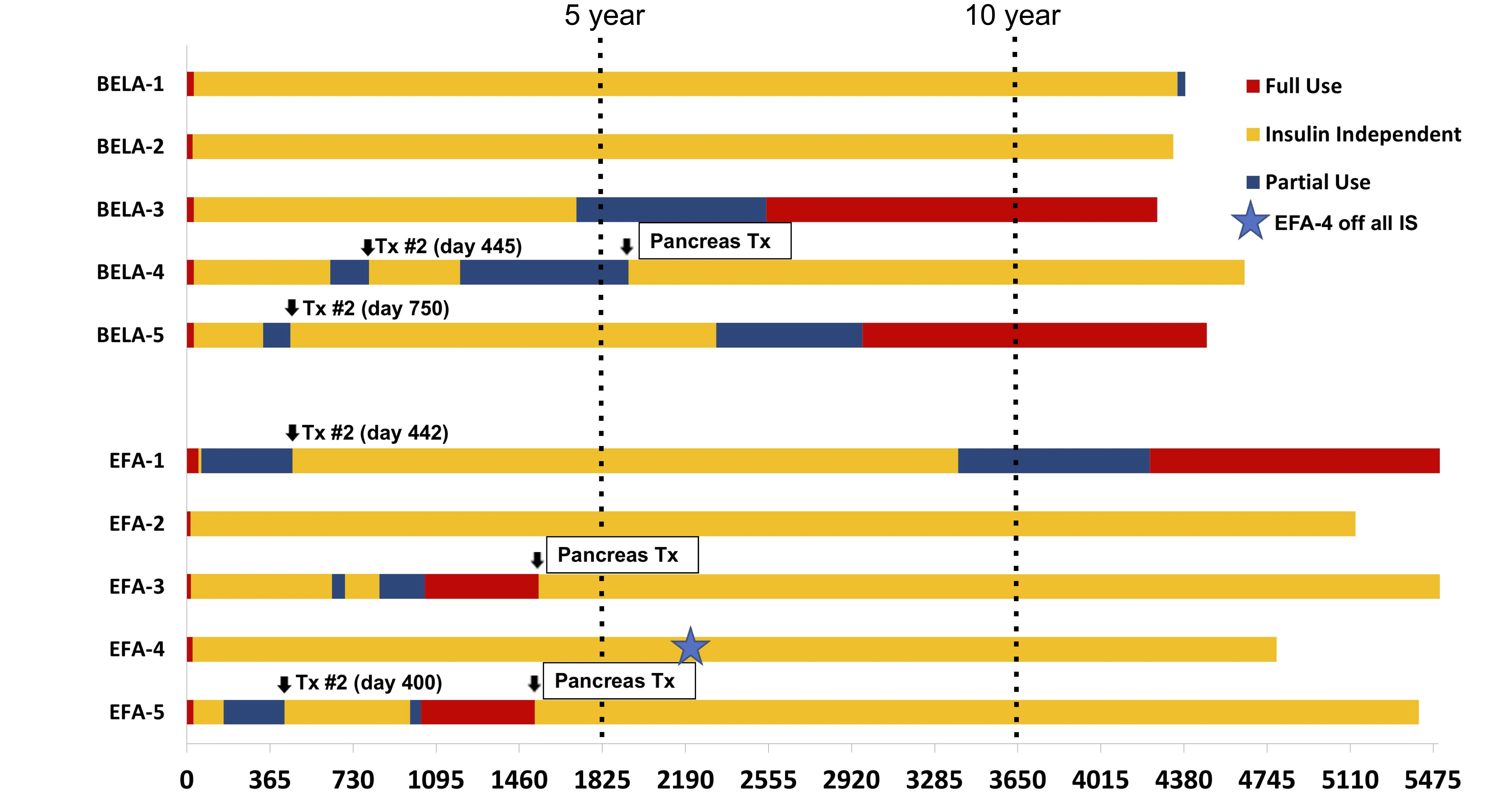
Results: Seven of 10 patients (70%) maintained insulin independence with normalization of HbA1c (3 BELA, 4 EFA) at 10 years post-islet transplant; four patients (2 EFA, 2 BELA) achieved long-term insulin independence after one islet infusion and three additional patients (2 EFA, 1 BELA) following PAI transplantation (Figure 1, Figure 2). Six of 10 (60%) remain insulin independent currently, at a mean follow-up of 13.3 ± 1.1 years from islet transplant. Eight of 10 patients (80%) maintained renal function with less than 30% decrease in GFR and without progression to stage 4 or stage 5 CKD (Figure 2). One patient progressed to stage 4 CKD following initiation of tacrolimus for PAI, and another progressed to stage 5 CKD after islet failure and return to full insulin dependence. Patients receiving EFA demonstrated profound expansion of circulating CD4+Foxp3+ regulatory T cells (Tregs). One patient (EFA-4) demonstrated Tregs comprising 68% of all circulating CD4+ T cells, and remains insulin independent despite discontinuing all immunosuppression, thus demonstrating the first reported case of operational tolerance in islet transplantation.
Conclusions: Our results describe a multi-modal, personalized approach to beta-cell replacement. In our series, repeat islet transplant is ineffective at maintaining long-term insulin independence. PAI results in durable insulin independence but is associated with deterioration of renal function secondary to CNI dependence. EFA-based immunosuppression resulted in significant expansion of Tregs following transplantation, with one islet recipient establishing operational tolerance.

Juvenile Diabetes Research Foundation (4-2004-372). NIH P30 (DK63720). NIH UO1 (AIO65193). CRC UL1 (RR024131). NIH T32 (AI125222).
