Oral antibiotics for treatment of gram negative bacteremia in solid organ transplant recipients: a retrospective observational study
Eliezer Zachary Nussbaum1, Camille N Kotton1.
1Infectious Diseases, Massachusetts General Hospital, Boston, MA, United States
Introduction: Treatment of gram-negative bloodstream infection (GNBI) with oral rather than intravenous (IV) antimicrobial agents can reduce length of stay, line associated complications and improve overall patient satisfaction. Data in immunocompetent subjects supports use of highly bioavailable oral agents for treatment of uncomplicated GNBI, however there is minimal literature regarding the safety and efficacy of this practice in solid organ transplant recipients (SOTR). We aimed to assess the safety and efficacy of oral for treatment of uncomplicated GNBI in SOTR.
Methods: We performed a retrospective observational study and identified all SOTR with GNBI within the Massachusetts General and Brigham and Women’s Hospital systems from 2016-2021. We then identified patients who were transitioned from intravenous to oral agents to complete their treatment. To be considered eligible for analysis, patients had to have adequate source control and could not have evidence of a complicated infection (e.g endocarditis, osteomyelitis, undrained abscess) that would inherently require a prolonged treatment course. We recorded patient demographics, type of and time from transplant, comorbidities and Charlson comorbidity score, PITT bacteremia score, immunosuppressive regimen, and eGFR at time of transition to an oral agent. We noted the source of infection, infecting organism, antimicrobial agents used and duration of oral and IV therapy. Primary endpoints were mortality, recurrence of bacteremia and re-initiation of IV antibiotics for the same underlying infectious process within 30 days of treatment completion. Secondary endpoints included length of stay, development of C difficile infection, treatment associated complications, and need for further oral antibiotics directed at the initial infectious process. We are collecting further data on a control population.
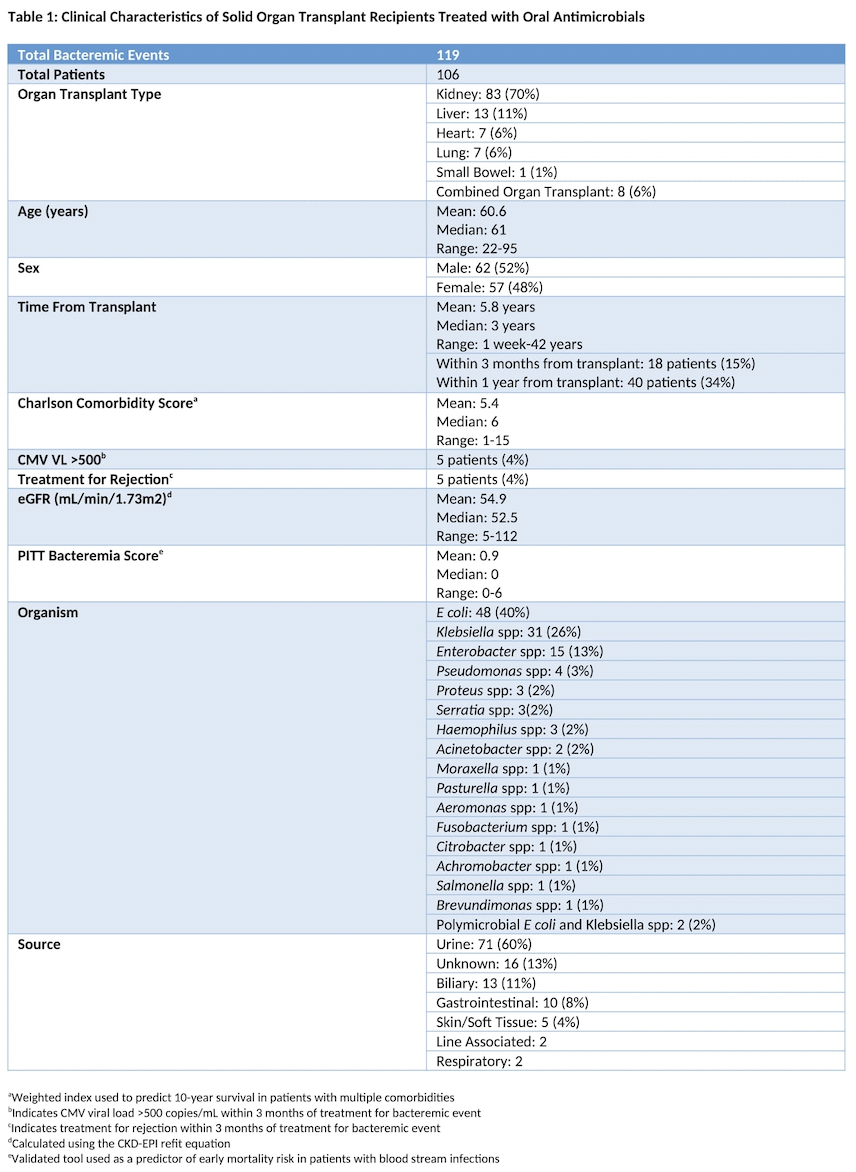
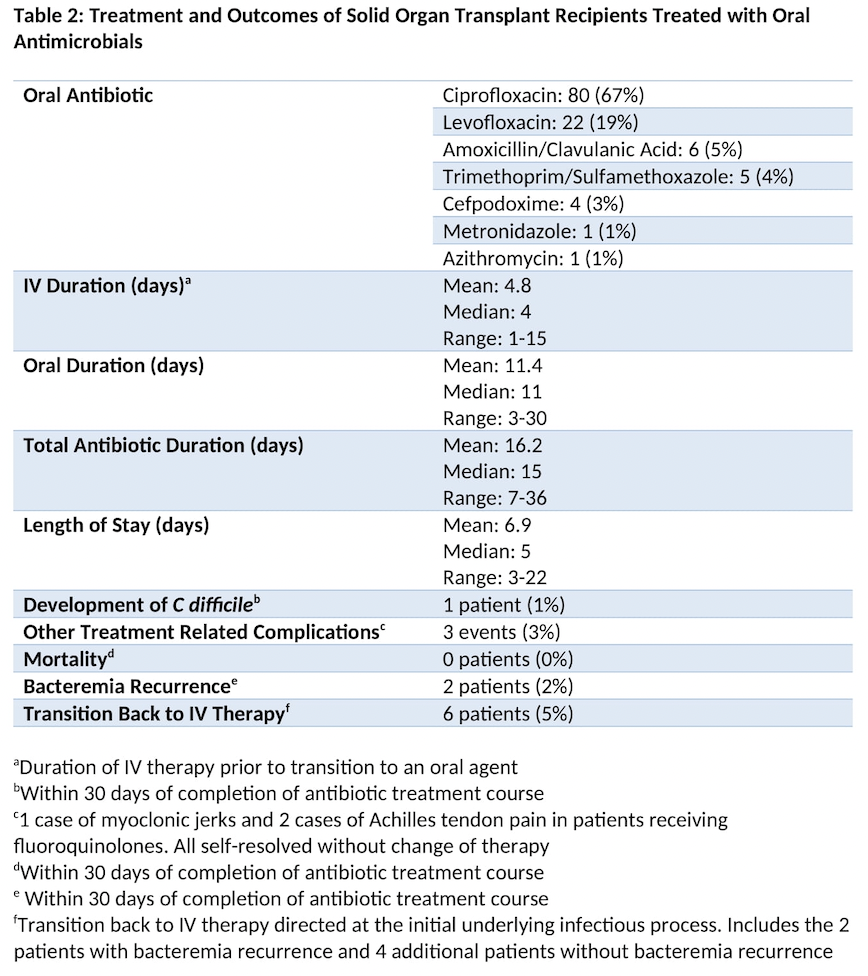
Results: 119 GNBIs from 106 patients met inclusion criteria. The most common transplant type was kidney (n=83). E. coli was the most frequently identified organism (n=50). The most common source was urinary (n=71) and the most commonly used oral antimicrobial was ciprofloxacin (n=80). Median duration of IV therapy prior to oral therapy was 4 days (range 1-15 days). Median duration of oral therapy was 11 days (range 3-30 days). Median time from transplant was 3 years (range 1 week – 42 years). Zero patients died within 30 days of treatment completion. 2 patients developed bacteremia recurrence within 30 days of treatment completion and 4 additional patients were transitioned back to IV therapy without bacteremia recurrence. Median length of hospital stay was 5 days. 1 patient developed C difficile. 3 patients developed oral therapy related complications that did not require treatment adjustment.
Conclusions: Conversion from IV to oral therapy with highly bioavailable antimicrobials is safe and effective for treatment of GNBI in SOTR. Randomized controlled trials are needed to confirm these findings.